X-ray Crystal Structure of Serine Hydroxymethyl Transferase from Burkholderia cenocepacia bound to PLP and Serine
Fairman, J.W., Jensen, M.M., Sullivan, A.H., Edwards, T.E., Lorimer, D.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Serine hydroxymethyltransferase | 423 | Burkholderia cenocepacia J2315 | Mutation(s): 0 Gene Names: glyA, BCAL3197 EC: 2.1.2.1 | 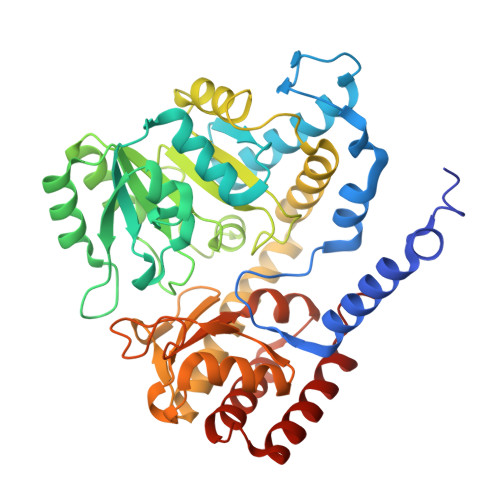 | |
UniProt | |||||
Find proteins for B4ECY9 (Burkholderia cenocepacia (strain ATCC BAA-245 / DSM 16553 / LMG 16656 / NCTC 13227 / J2315 / CF5610)) Explore B4ECY9 Go to UniProtKB: B4ECY9 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | B4ECY9 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| PLP Query on PLP | E [auth A], H [auth B], K [auth C], L [auth D] | PYRIDOXAL-5'-PHOSPHATE C8 H10 N O6 P NGVDGCNFYWLIFO-UHFFFAOYSA-N |  | ||
| SER Query on SER | F [auth A], G [auth A], I [auth B], J [auth B] | SERINE C3 H7 N O3 MTCFGRXMJLQNBG-REOHCLBHSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 66.22 | α = 90 |
| b = 179.84 | β = 115.25 |
| c = 75.64 | γ = 90 |
| Software Name | Purpose |
|---|---|
| XSCALE | data scaling |
| PHASER | phasing |
| PHENIX | refinement |
| PDB_EXTRACT | data extraction |