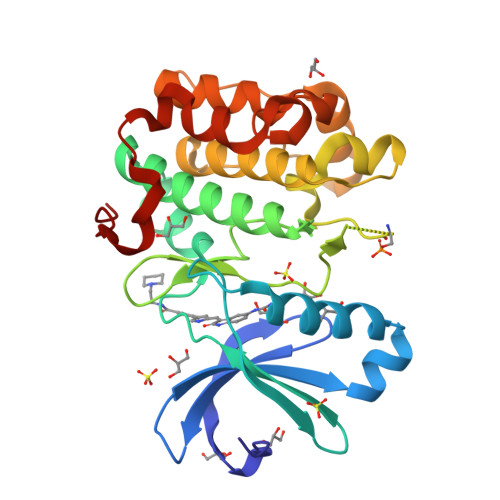
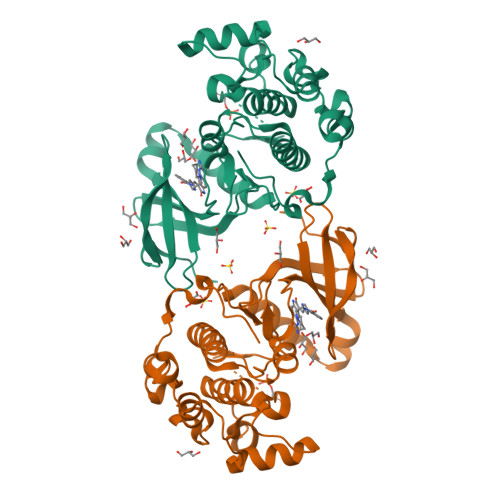
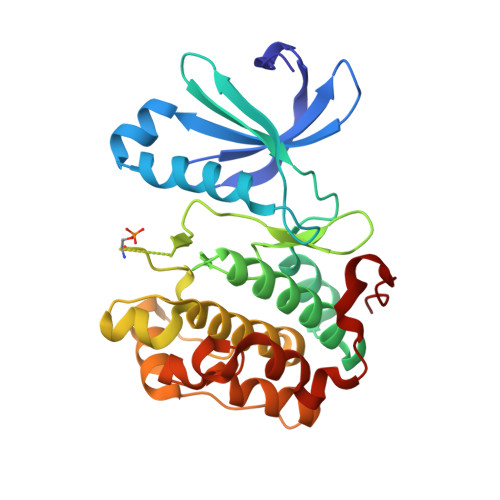
Indolinone based phosphoinositide-dependent kinase-1 (PDK1) inhibitors. Part 2: Optimization of BX-517.
Islam, I., Brown, G., Bryant, J., Hrvatin, P., Kochanny, M.J., Phillips, G.B., Yuan, S., Adler, M., Whitlow, M., Lentz, D., Polokoff, M.A., Wu, J., Shen, J., Walters, J., Ho, E., Subramanyam, B., Zhu, D., Feldman, R.I., Arnaiz, D.O.(2007) Bioorg Med Chem Lett 17: 3819-3825
- PubMed: 17544272
- DOI: https://doi.org/10.1016/j.bmcl.2007.05.060
- Primary Citation of Related Structures:
2PE2 - PubMed Abstract:
Based on the lead compound BX-517, a series of C-4' substituted indolinones have been synthesized and evaluated for PDK1 inhibition. Modification at C-4' of the pyrrole afforded potent compounds (7b and 7d) with improved solubility and ADME properties. In this letter, we describe the synthesis, selectivity profile, and pharmacokinetic data of selected compounds.
Organizational Affiliation:
Berlex Biosciences, 2600 Hilltop Dr. Richmond, CA 94804, USA. imadulislam@yahoo.com