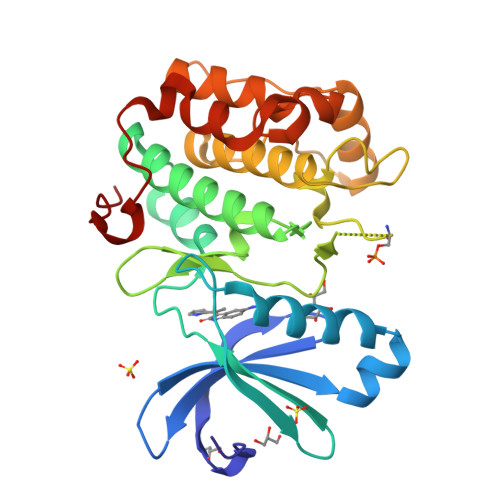
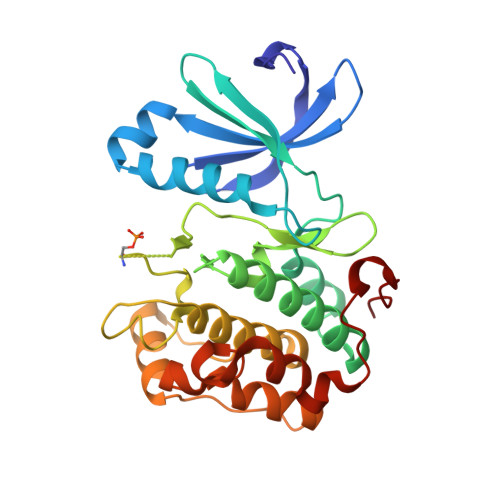
Indolinone based phosphoinositide-dependent kinase-1 (PDK1) inhibitors. Part 1: Design, synthesis and biological activity.
Islam, I., Bryant, J., Chou, Y.L., Kochanny, M.J., Lee, W., Phillips, G.B., Yu, H., Adler, M., Whitlow, M., Ho, E., Lentz, D., Polokoff, M.A., Subramanyam, B., Wu, J.M., Zhu, D., Feldman, R.I., Arnaiz, D.O.(2007) Bioorg Med Chem Lett 17: 3814-3818
- PubMed: 17531483
- DOI: https://doi.org/10.1016/j.bmcl.2007.04.071
- Primary Citation of Related Structures:
2PE0, 2PE1 - PubMed Abstract:
HTS screening identified 1 with micromolar inhibitory activity against PDK1. Optimization of 1 afforded 4i (BX-517) which has single-digit nanomolar activity against PDK1 and excellent selectivity against PKA.
Organizational Affiliation:
Berlex Biosciences, 2600 Hilltop Dr. Richmond, CA 94804, USA.