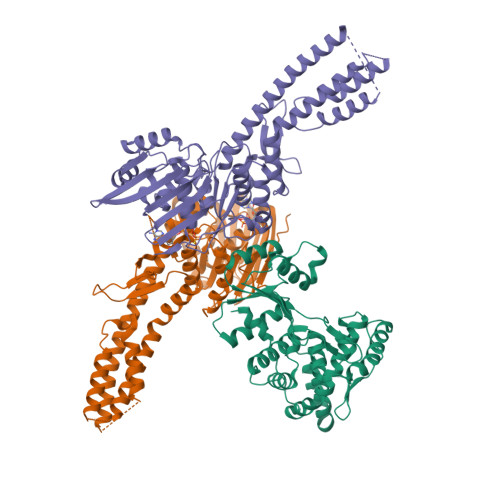
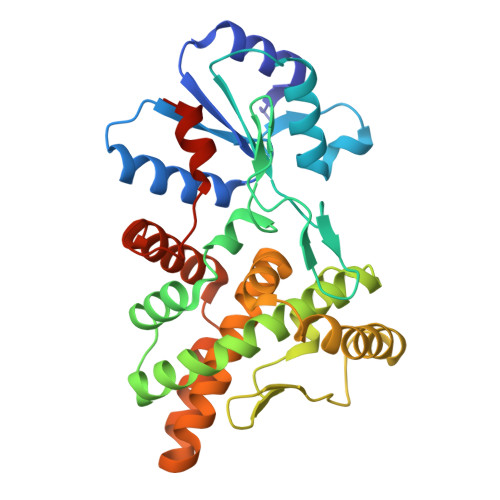
A virally encoded tRNA neutralizes the PARIS antiviral defence system.
Burman, N., Belukhina, S., Depardieu, F., Wilkinson, R.A., Skutel, M., Santiago-Frangos, A., Graham, A.B., Livenskyi, A., Chechenina, A., Morozova, N., Zahl, T., Henriques, W.S., Buyukyoruk, M., Rouillon, C., Saudemont, B., Shyrokova, L., Kurata, T., Hauryliuk, V., Severinov, K., Groseille, J., Thierry, A., Koszul, R., Tesson, F., Bernheim, A., Bikard, D., Wiedenheft, B., Isaev, A.(2024) Nature 634: 424-431
- PubMed: 39111359
- DOI: https://doi.org/10.1038/s41586-024-07874-3
- Primary Citation of Related Structures:
8UX9 - PubMed Abstract:
Viruses compete with each other for limited cellular resources, and some deliver defence mechanisms that protect the host from competing genetic parasites 1 . The phage antirestriction induced system (PARIS) is a defence system, often encoded in viral genomes, that is composed of a 55 kDa ABC ATPase (AriA) and a 35 kDa TOPRIM nuclease (AriB) 2 . However, the mechanism by which AriA and AriB function in phage defence is unknown. Here we show that AriA and AriB assemble into a 425 kDa supramolecular immune complex. We use cryo-electron microscopy to determine the structure of this complex, thereby explaining how six molecules of AriA assemble into a propeller-shaped scaffold that coordinates three subunits of AriB. ATP-dependent detection of foreign proteins triggers the release of AriB, which assembles into a homodimeric nuclease that blocks infection by cleaving host lysine transfer RNA. Phage T5 subverts PARIS immunity through expression of a lysine transfer RNA variant that is not cleaved by PARIS, thereby restoring viral infection. Collectively, these data explain how AriA functions as an ATP-dependent sensor that detects viral proteins and activates the AriB toxin. PARIS is one of an emerging set of immune systems that form macromolecular complexes for the recognition of foreign proteins, rather than foreign nucleic acids 3 .
Organizational Affiliation:
Department of Microbiology and Cell Biology, Montana State University, Bozeman, MT, USA.