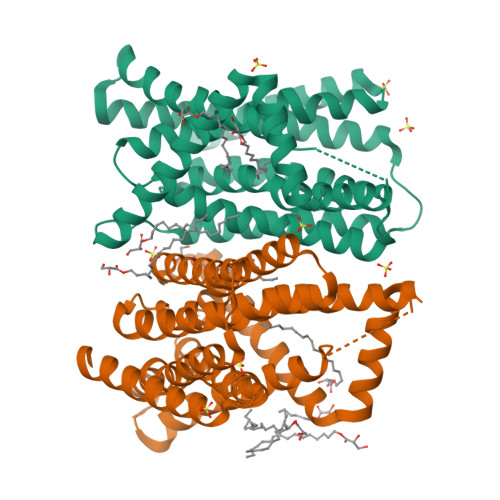
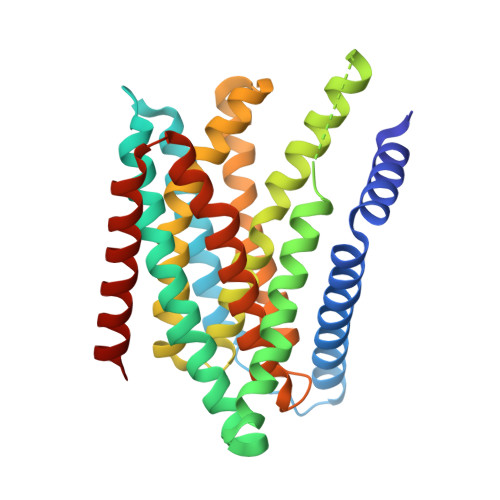
Structural insights into the elevator-type transport mechanism of a bacterial ZIP metal transporter.
Zhang, Y., Jiang, Y., Gao, K., Sui, D., Yu, P., Su, M., Wei, G.W., Hu, J.(2023) Nat Commun 14: 385-385
- PubMed: 36693843
- DOI: https://doi.org/10.1038/s41467-023-36048-4
- Primary Citation of Related Structures:
8CZJ - PubMed Abstract:
The Zrt-/Irt-like protein (ZIP) family consists of ubiquitously expressed divalent metal transporters critically involved in maintaining systemic and cellular homeostasis of zinc, iron, and manganese. Here, we present a study on a prokaryotic ZIP from Bordetella bronchiseptica (BbZIP) by combining structural biology, evolutionary covariance, computational modeling, and a variety of biochemical assays to tackle the issue of the transport mechanism which has not been established for the ZIP family. The apo state structure in an inward-facing conformation revealed a disassembled transport site, altered inter-helical interactions, and importantly, a rigid body movement of a 4-transmembrane helix (TM) bundle relative to the other TMs. The computationally generated and biochemically validated outward-facing conformation model revealed a slide of the 4-TM bundle, which carries the transport site(s), by approximately 8 Å toward the extracellular side against the static TMs which mediate dimerization. These findings allow us to conclude that BbZIP is an elevator-type transporter.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, Michigan State University, East Lansing, MI, USA.