Blm10-Based Compounds Add to the Knowledge of How Allosteric Modulators Influence Human 20S Proteasome.
Witkowska, J., Gizynska, M., Karpowicz, P., Sowik, D., Trepczyk, K., Hennenberg, F., Chari, A., Gieldon, A., Pierzynowska, K., Gaffke, L., Wegrzyn, G., Jankowska, E.(2025) ACS Chem Biol
- PubMed: 39907714
- DOI: https://doi.org/10.1021/acschembio.4c00341
- Primary Citation of Related Structures:
8BZL - PubMed Abstract:
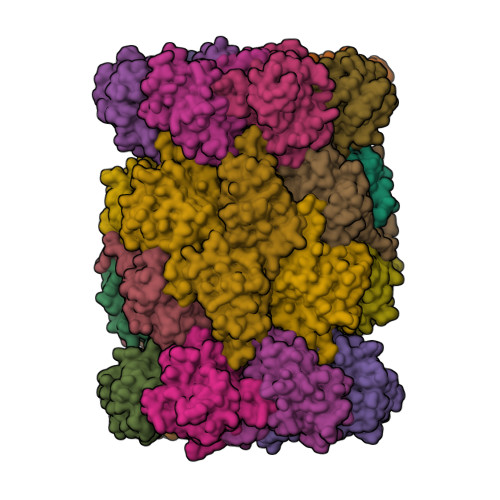
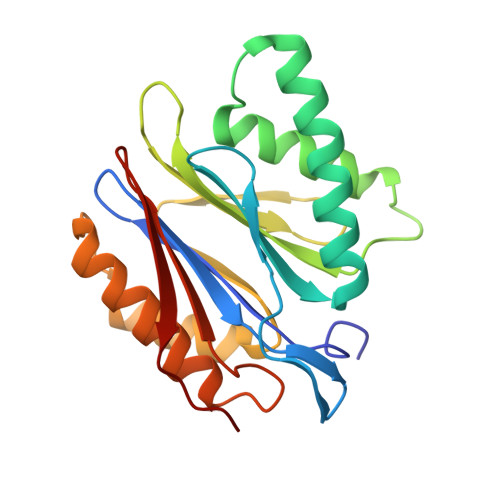
Proteasomes catalyze protein degradation in cells and play an integral role in cellular homeostasis. Its activity decreases with age alongside the load of defective proteins, resulting from mutations or oxidative stress-induced damage. Such proteins are prone to aggregation and, if not efficiently degraded, can form toxic oligomers and amyloid plaques. Developing an effective way to activate the proteasome could prevent such pathologies. Designing activators is not easy because they do not bind in the active site, which is well-defined and highly conserved, but away from it. The structures of proteasome complexes with natural activators can help here, but these are large proteins, some even multimeric, whose activity is difficult to replace with a small-molecule compound. Nevertheless, the use of fragments of such proteins makes it possible to accumulate knowledge about the relevance of various structural elements for efficient and selective activation. Here, we presented peptidic activators of the 20S proteasome, which were designed based on both the C -terminal sequence of the yeast proteasome activator, Blm10 protein, and the interactions predicted by molecular modeling. These Blm analogs were able to stimulate human 20S proteasome to more efficiently degrade both small fluorogenic substrates and proteins. The best activators also demonstrated their efficacy in cell lysates. X-ray crystallography indicated that an effective modulator can bind to several sites on the surface of the proteasome without causing permanent structural changes in its immediate vicinity but affecting the active sites.
Organizational Affiliation:
Department of Biomedical Chemistry, Faculty of Chemistry, University of Gdańsk, Gdańsk 80-308, Poland.