Chemoproteomics-Enabled Identification of 4-Oxo-beta-Lactams as Inhibitors of Dipeptidyl Peptidases 8 and 9.
Carvalho, L.A.R., Ross, B., Fehr, L., Bolgi, O., Wohrle, S., Lum, K.M., Podlesainski, D., Vieira, A.C., Kiefersauer, R., Felix, R., Rodrigues, T., Lucas, S.D., Gross, O., Geiss-Friedlander, R., Cravatt, B.F., Huber, R., Kaiser, M., Moreira, R.(2022) Angew Chem Int Ed Engl 61: e202210498-e202210498
- PubMed: 36089535
- DOI: https://doi.org/10.1002/anie.202210498
- Primary Citation of Related Structures:
7A3G, 7A3J, 7A3L, 7AYQ, 7AYR, 7OR4, 7OZ7, 7ZXS - PubMed Abstract:
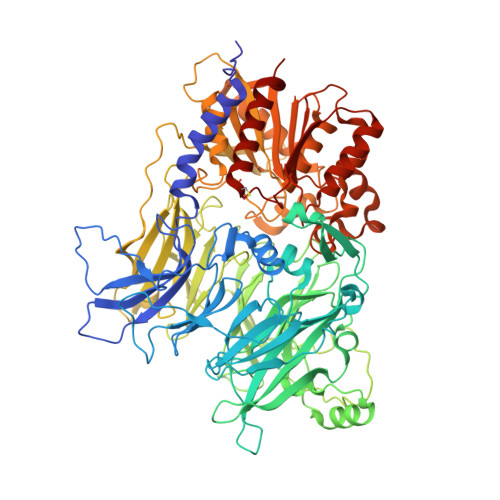
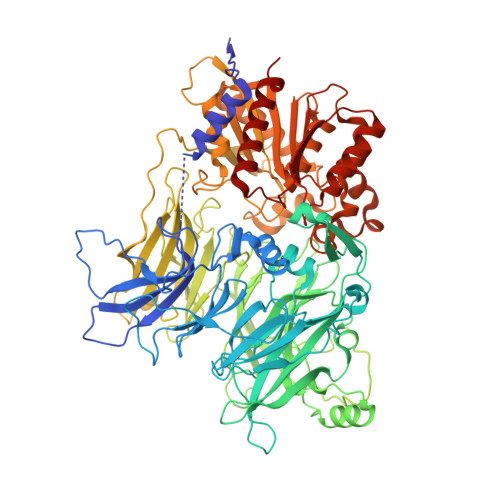
Dipeptidyl peptidases 8 and 9 (DPP8/9) have gathered interest as drug targets due to their important roles in biological processes like immunity and tumorigenesis. Elucidation of their distinct individual functions remains an ongoing task and could benefit from the availability of novel, chemically diverse and selective chemical tools. Here, we report the activity-based protein profiling (ABPP)-mediated discovery of 4-oxo-β-lactams as potent, non-substrate-like nanomolar DPP8/9 inhibitors. X-ray crystallographic structures revealed different ligand binding modes for DPP8 and DPP9, including an unprecedented targeting of an extended S2' (eS2') subsite in DPP8. Biological assays confirmed inhibition at both target and cellular levels. Altogether, our integrated chemical proteomics and structure-guided small molecule design approach led to novel DPP8/9 inhibitors with alternative molecular inhibition mechanisms, delivering the highest selectivity index reported to date.
Organizational Affiliation:
Department of Pharmaceutical Sciences and Medicines, Research Institute for Medicines (iMed.ULisboa), Faculdade de Farmácia, Universidade de Lisboa, Av. Prof. Gama Pinto, 1649-003, Lisboa, Portugal.