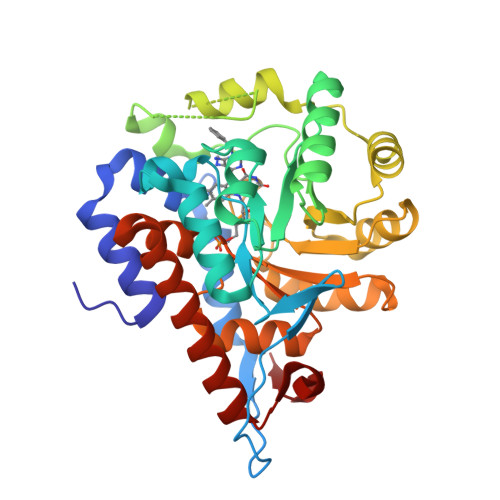
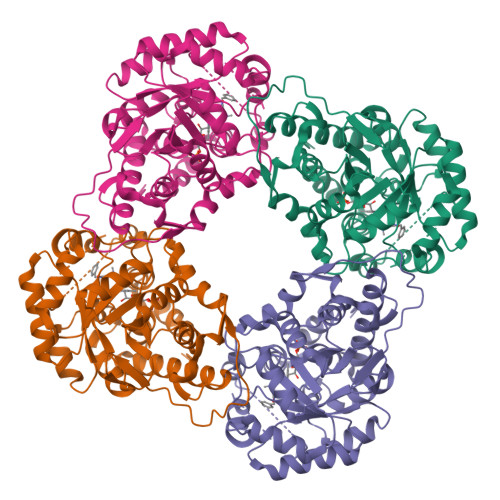
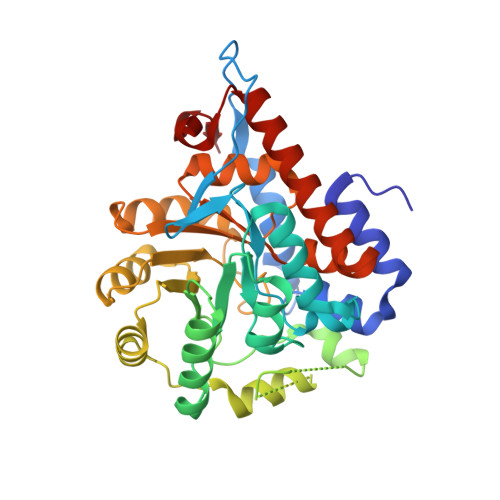
Structure of human hydroxyacid oxidase 1 bound with 5-bromo-N-methyl-1H-indazole-3-carboxamide
Mackinnon, S., Bezerra, G.A., Krojer, T., Bradley, A.R., Talon, R., Brandeo-Neto, J., Douangamath, A., von Delft, F., Arrowsmith, C.H., Edwards, A., Bountra, C., Oppermann, U., Brennan, P.E., Yue, W.W.To be published.