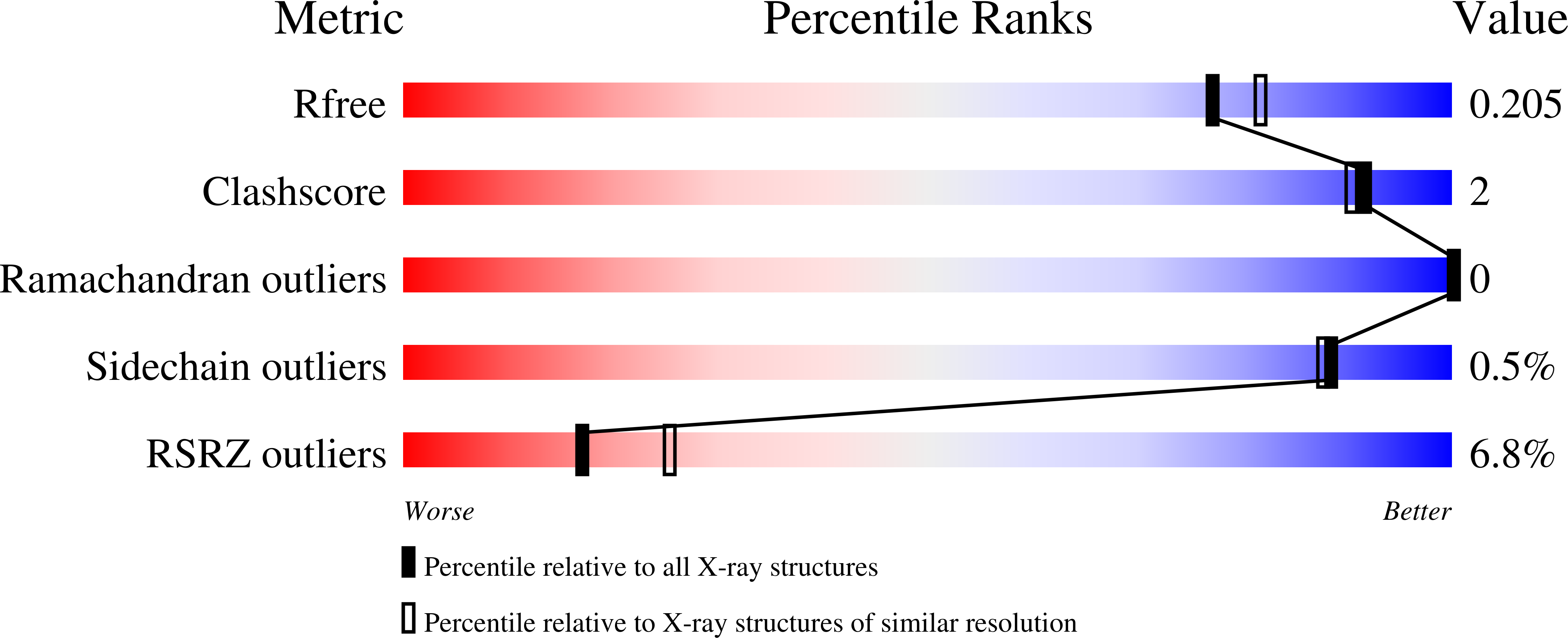
Targeting SARS-CoV-2 Nsp3 macrodomain structure with insights from human poly(ADP-ribose) glycohydrolase (PARG) structures with inhibitors.
Brosey, C.A., Houl, J.H., Katsonis, P., Balapiti-Modarage, L.P.F., Bommagani, S., Arvai, A., Moiani, D., Bacolla, A., Link, T., Warden, L.S., Lichtarge, O., Jones, D.E., Ahmed, Z., Tainer, J.A.(2021) Prog Biophys Mol Biol 163: 171-186
- PubMed: 33636189
- DOI: https://doi.org/10.1016/j.pbiomolbio.2021.02.002
- Primary Citation of Related Structures:
7KFP, 7KG0, 7KG1, 7KG3, 7KG6, 7KG7, 7KG8, 7KXB, 7LG7 - PubMed Abstract:
Arrival of the novel SARS-CoV-2 has launched a worldwide effort to identify both pre-approved and novel therapeutics targeting the viral proteome, highlighting the urgent need for efficient drug discovery strategies. Even with effective vaccines, infection is possible, and at-risk populations would benefit from effective drug compounds that reduce the lethality and lasting damage of COVID-19 infection. The CoV-2 MacroD-like macrodomain (Mac1) is implicated in viral pathogenicity by disrupting host innate immunity through its mono (ADP-ribosyl) hydrolase activity, making it a prime target for antiviral therapy. We therefore solved the structure of CoV-2 Mac1 from non-structural protein 3 (Nsp3) and applied structural and sequence-based genetic tracing, including newly determined A. pompejana MacroD2 and GDAP2 amino acid sequences, to compare and contrast CoV-2 Mac1 with the functionally related human DNA-damage signaling factor poly (ADP-ribose) glycohydrolase (PARG). Previously, identified targetable features of the PARG active site allowed us to develop a pharmacologically useful PARG inhibitor (PARGi). Here, we developed a focused chemical library and determined 6 novel PARGi X-ray crystal structures for comparative analysis. We applied this knowledge to discovery of CoV-2 Mac1 inhibitors by combining computation and structural analysis to identify PARGi fragments with potential to bind the distal-ribose and adenosyl pockets of the CoV-2 Mac1 active site. Scaffold development of these PARGi fragments has yielded two novel compounds, PARG-345 and PARG-329, that crystallize within the Mac1 active site, providing critical structure-activity data and a pathway for inhibitor optimization. The reported structural findings demonstrate ways to harness our PARGi synthesis and characterization pipeline to develop CoV-2 Mac1 inhibitors targeting the ADP-ribose active site. Together, these structural and computational analyses reveal a path for accelerating development of antiviral therapeutics from pre-existing drug optimization pipelines.
Organizational Affiliation:
Department of Molecular and Cellular Oncology, M. D. Anderson Cancer Center, Houston, TX, 77030, USA. Electronic address: [email protected].