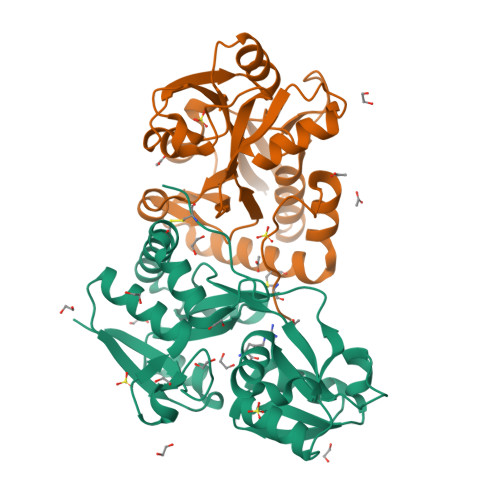
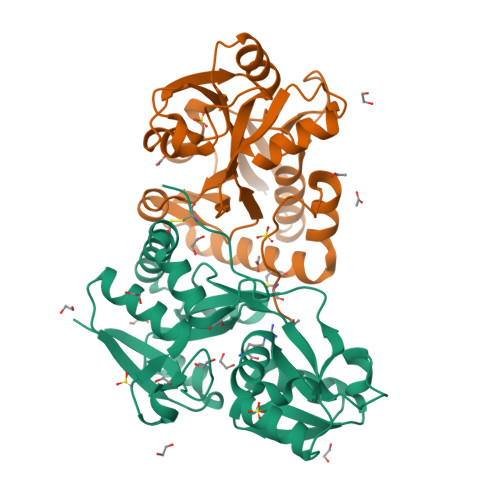
Crystal structures of a putative periplasmic cystine-binding protein from Candidatus Liberibacter asiaticus: insights into an adapted mechanism of ligand binding.
Kumar, P., Kesari, P., Kokane, S., Ghosh, D.K., Kumar, P., Sharma, A.K.(2019) FEBS J 286: 3450-3472
- PubMed: 31063259
- DOI: https://doi.org/10.1111/febs.14921
- Primary Citation of Related Structures:
6A80, 6A8S, 6AA1, 6AAL - PubMed Abstract:
The amino acid-binding receptors, a component of ABC transporters, have evolved to cater to different specificities and functions. Of particular interest are cystine-binding receptors, which have shown broad specificity. In the present study, a putative periplasmic cystine-binding protein from Candidatus Liberibacter asiaticus (CLasTcyA) was characterized. Analysis of the CLasTcyA sequence and crystal structures in the ligand-bound state revealed novel features of CLasTcyA in comparison to related proteins. One of the unique features found in CLasTcyA structure was the positioning of the C-terminal extended loop of one chain very close to the substrate-binding site of the adjacent monomer in the asymmetric unit. The presence of a disulphide bond, unique to Candidatus Liberibacter family, holds the C-terminal extended loop in position. Analysis of the substrate-binding pocket of CLasTcyA suggested a broad specificity and a completely different orientation of the bound substrates in comparison to related protein structures. The open conformation for one of the two chains of the asymmetric unit in the Arg-bound structure revealed a limited open state (18.4°) for CLasTcyA as compared to open state of other related proteins (~ 60°). The strong interaction between Asp126 on helix-α5 of small domain and Arg82 (bigger domain) restricts the degree of opening in ligand-free open state. The dissociation constant of 1.26 μm by SPR and 3.7 μm by MST exhibited low affinity for the cystine. This is the first structural characterization of an l-cystine ABC transporter from plant pathogen and our results suggest that CLasTcyA may have evolved to cater to its specific needs for its survival in the host.
Organizational Affiliation:
Department of Biotechnology, Indian Institute of Technology Roorkee, India.