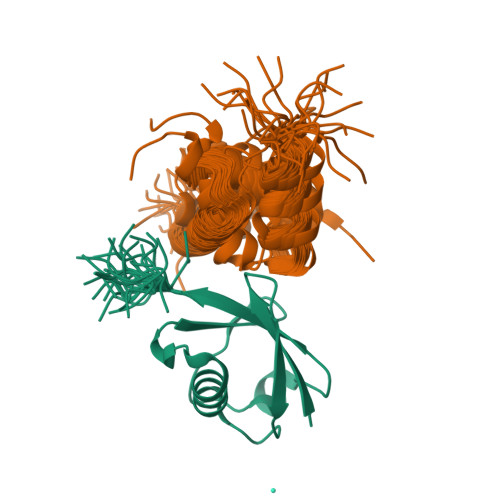
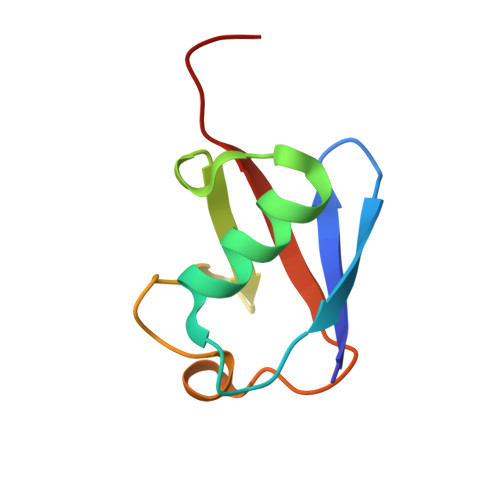
Lanthanoid tagging via an unnatural amino acid for protein structure characterization
Jiang, W.X., Gu, X.H., Dong, X., Tang, C.(2017) J Biomol NMR 67: 273-282
- PubMed: 28365903
- DOI: https://doi.org/10.1007/s10858-017-0106-9
- Primary Citation of Related Structures:
5XBO - PubMed Abstract:
Lanthanoid pseudo-contact shift (PCS) provides long-range structural information between a paramagnetic tag and protein nuclei. However, for proteins with native cysteines, site-specific attachment may only utilize functional groups orthogonal to sulfhydryl chemistry. Here we report two lanthanoid probes, DTTA-C3-yne and DTTA-C4-yne, which can be conjugated to an unnatural amino acid pAzF in the target protein via azide-alkyne cycloaddition. Demonstrated with ubiquitin and cysteine-containing enzyme EIIB, we show that large PCSs of distinct profiles can be generated for each tag/lanthanoid combination. The DTTA-based lanthanoid tags are associated with large magnetic susceptibility tensors owing to the rigidity of the tags. In particular, introduction of the DTTA-C3 tag affords intermolecular PCSs and enables structural characterization of a transient protein complex between ubiquitin and a UBA domain. Together, we have expanded the repertoire of paramagnetic tags and the applicability of paramagnetic NMR.
Organizational Affiliation:
CAS Key Laboratory of Magnetic Resonance in Biological Systems, State Key Laboratory of Magnetic Resonance and Atomic Molecular Physics, National Center for Magnetic Resonance at Wuhan, Collaborative Innovation Center of Chemistry for Life Sciences, Wuhan Institute of Physics and Mathematics of the Chinese Academy of Sciences, Wuhan, 430071, Hubei, China.