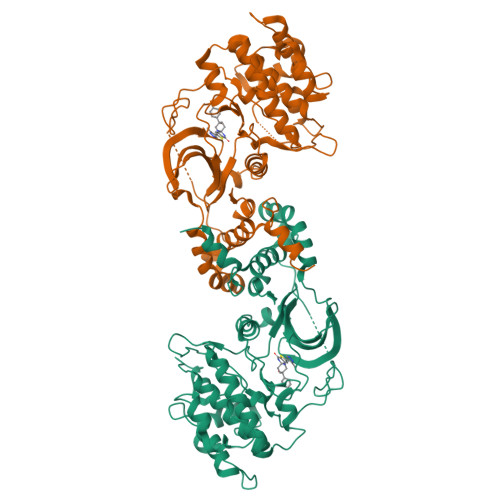
Identification of a new class of potent Cdc7 inhibitors designed by putative pharmacophore model: Synthesis and biological evaluation of 2,3-dihydrothieno[3,2-d]pyrimidin-4(1H)-ones.
Kurasawa, O., Oguro, Y., Miyazaki, T., Homma, M., Mori, K., Iwai, K., Hara, H., Skene, R., Hoffman, I., Ohashi, A., Yoshida, S., Ishikawa, T., Cho, N.(2017) Bioorg Med Chem 25: 2133-2147
- PubMed: 28284870
- DOI: https://doi.org/10.1016/j.bmc.2017.02.021
- Primary Citation of Related Structures:
5U7Q, 5U7R - PubMed Abstract:
Cell division cycle 7 (Cdc7) is a serine/threonine kinase that plays important roles in the regulation of DNA replication process. A genetic study indicates that Cdc7 inhibition can induce selective tumor-cell death in a p53-dependent manner, suggesting that Cdc7 is an attractive target for the treatment of cancers. In order to identify a new class of potent Cdc7 inhibitors, we generated a putative pharmacophore model based on in silico docking analysis of a known inhibitor with Cdc7 homology model. The pharmacophore model provided a minimum structural motif of Cdc7 inhibitor, by which preliminary medicinal chemistry efforts identified a dihydrothieno[3,2-d]-pyrimidin-4(1H)-one scaffold having a heteroaromatic hinge-binding moiety. The structure-activity relationship (SAR) studies resulted in the discovery of new, potent, and selective Cdc7 inhibitors 14a, c, e. Furthermore, the high selectivity of 14c, e for Cdc7 over Rho-associated protein kinase 1 (ROCK1) is discussed by utilizing a docking study with Cdc7 and ROCK2 crystal structures.
Organizational Affiliation:
Pharmaceutical Research Division, Takeda Pharmaceutical Company, Ltd., 26-1, Muraoka-Higashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Electronic address: osamu.kurasawa@takeda.com.