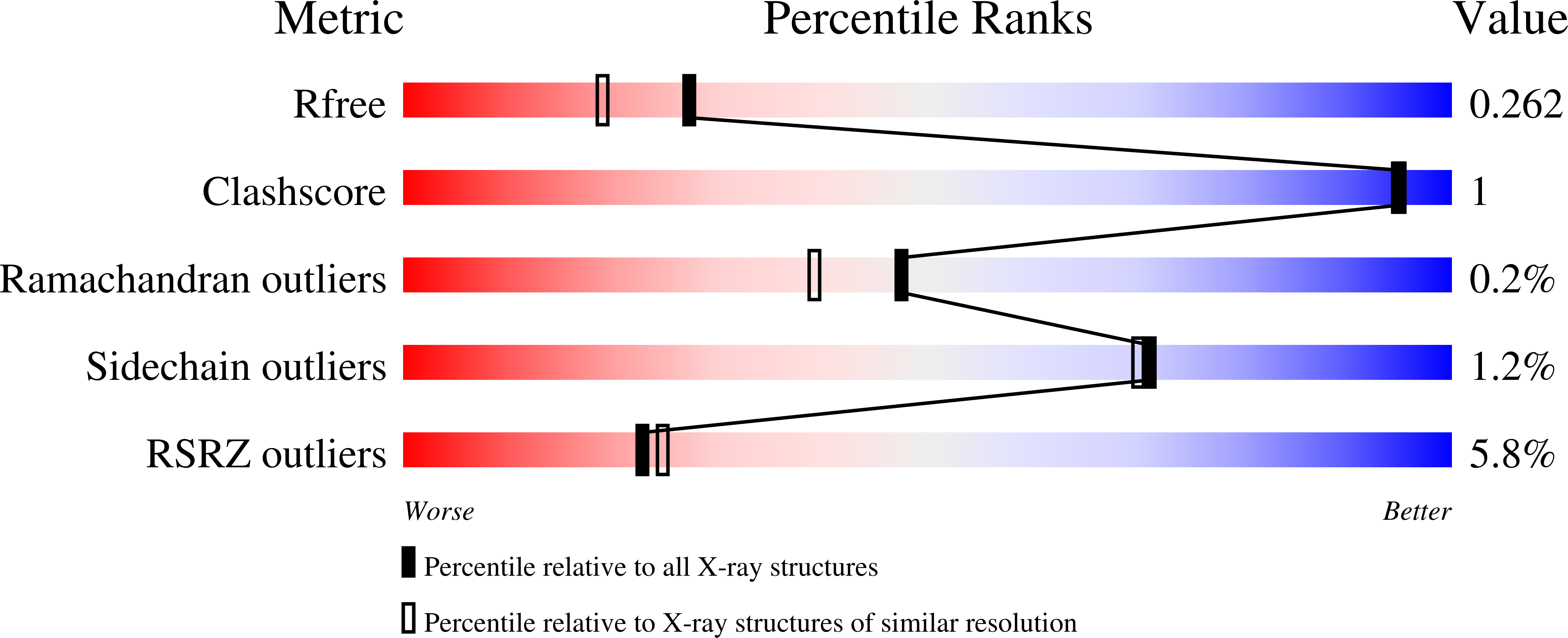
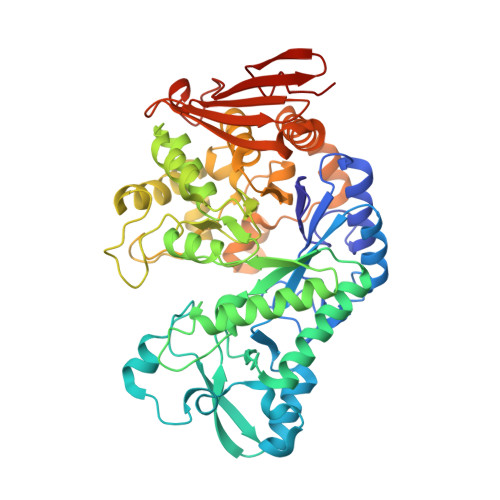
Switching enzyme specificity from phosphate to resveratrol glucosylation.
Kraus, M., Grimm, C., Seibel, J.(2017) Chem Commun (Camb) 53: 12181-12184
- PubMed: 29057405
- DOI: https://doi.org/10.1039/c7cc05993k
- Primary Citation of Related Structures:
5M9X, 5MAN - PubMed Abstract:
Here we present a point mutation-triggered domain shift which switches the acceptor preference of a sucrose phosphorylase from phosphate to a variety of large polyphenolic compounds including resveratrol and quercetin, enabling their efficient glucosylation. The variant possesses a high affinity for aromatic substrates due to newly introduced π-π- and hydrophobic interactions in the altered active site. The domain shift brings about a substantially enlarged and multifunctional active site for polyphenol glucosylation and rare disaccharide production. The crystal structure of the variant with its product resveratrol-3-α-d-glucoside allows the prediction of the substrate scope and regioselectivity of the aromatic compounds' glucosylation sites.
Organizational Affiliation:
Department of Organic Chemistry, Universität Würzburg, Am Hubland, 97074 Würzburg, Germany. [email protected].