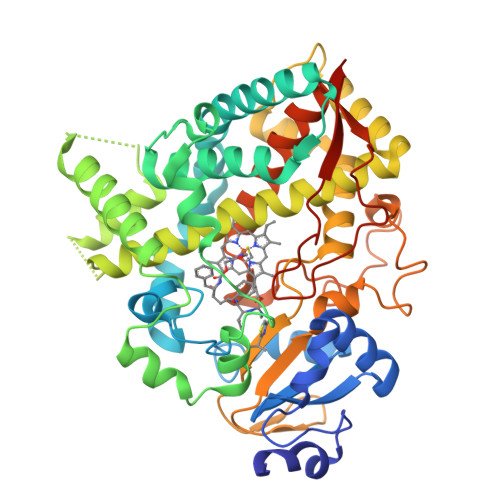
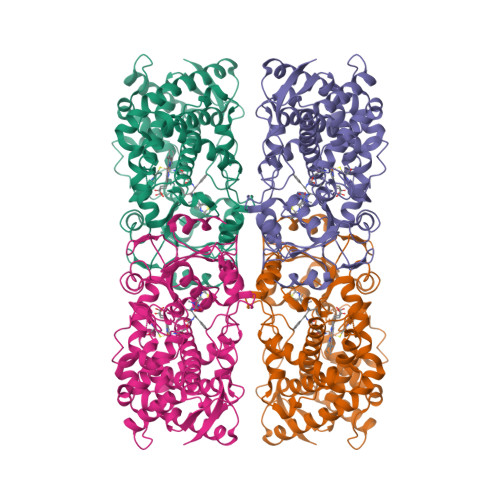
Pyridine-Substituted Desoxyritonavir Is a More Potent Inhibitor of Cytochrome P450 3A4 than Ritonavir.
Sevrioukova, I.F., Poulos, T.L.(2013) J Med Chem 56: 3733-3741
- PubMed: 23586711
- DOI: https://doi.org/10.1021/jm400288z
- Primary Citation of Related Structures:
4I3Q, 4I4G, 4I4H - PubMed Abstract:
Utilization of the cytochrome P450 3A4 (CYP3A4) inhibitor ritonavir as a pharmacoenhancer for anti-HIV drugs revolutionized the treatment of HIV infection. However, owing to ritonavir-related complications, there is a need for development of new CYP3A4 inhibitors with improved pharmacochemical properties, which requires a full understanding of the CYP3A4 inactivation mechanisms and the unraveling of possible inhibitor binding modes. We investigated the mechanism of CYP3A4 interaction with three desoxyritonavir analogues, containing the heme-ligating imidazole, oxazole, or pyridine group instead of the thiazole moiety (compounds 1, 2, and 3, respectively). Our data show that compound 3 is superior to ritonavir in terms of binding affinity and inhibitory potency owing to greater flexibility and the ability to adopt a conformation that minimizes steric clashing and optimizes protein-ligand interactions. Additionally, Ser119 was identified as a key residue assisting binding of ritonavir-like inhibitors, which emphasizes the importance of polar interactions in the CYP3A4-ligand association.
Organizational Affiliation:
Departments of Molecular Biology and Biochemistry, University of California, Irvine, California 92697, United States. sevrioui@uci.edu