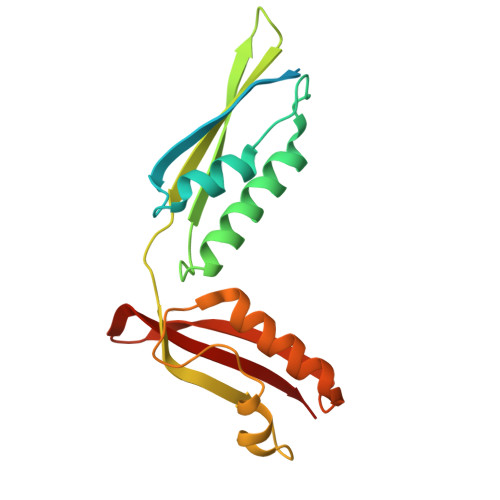
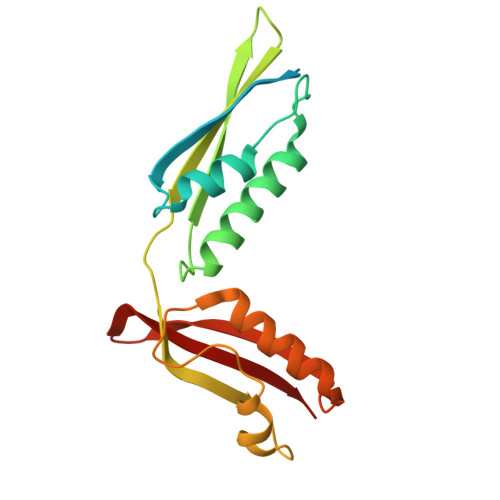
High-resolution structure of a new crystal form of BamA POTRA4-5 from Escherichia coli.
Zhang, H., Gao, Z.Q., Hou, H.F., Xu, J.H., Li, L.F., Su, X.D., Dong, Y.H.(2011) Acta Crystallogr Sect F Struct Biol Cryst Commun 67: 734-738
- PubMed: 21795783
- DOI: https://doi.org/10.1107/S1744309111014254
- Primary Citation of Related Structures:
3Q6B - PubMed Abstract:
In Escherichia coli, the BAM complex is employed to mediate correct folding of the outer membrane (OM) proteins into β-barrels and their insertion into the OM. BamA, which is an essential component of the complex, consists of a C-terminal transmembrane region and five N-terminal polypeptide transport-associated (POTRA) domains. Although deletion studies have shown that each of the POTRA domains plays an important role in the process of BAM complex formation, only POTRA5 is essential for cell viability. Here, the crystal structure of POTRA4-5 has been determined to 1.50 Å resolution with an R factor of 14.7% and an Rfree of 18.9%.
Organizational Affiliation:
National Laboratory of Protein Engineering and Plant Genetic Engineering, College of Life Science, Peking University, Beijing 100871, People's Republic of China.