Crystal structure of enoyl-acyl carrier protein reductase (FabK) from Streptococcus pneumoniae reveals the binding mode of an inhibitor.
Saito, J., Yamada, M., Watanabe, T., Iida, M., Kitagawa, H., Takahata, S., Ozawa, T., Takeuchi, Y., Ohsawa, F.(2008) Protein Sci 17: 691-699
- PubMed: 18305197
- DOI: https://doi.org/10.1110/ps.073288808
- Primary Citation of Related Structures:
2Z6I, 2Z6J - PubMed Abstract:
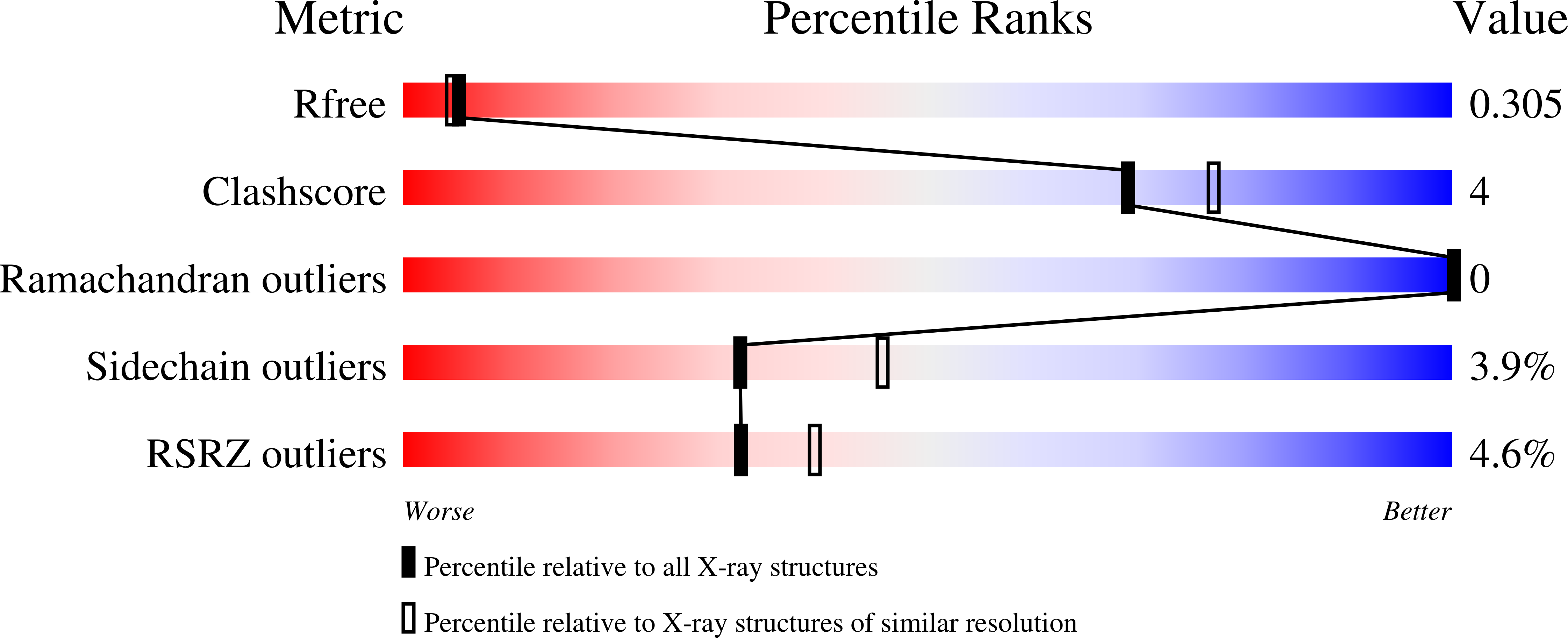
Enoyl-acyl carrier protein (ACP) reductases are critical for bacterial type II fatty acid biosynthesis and thus are attractive targets for developing novel antibiotics. We determined the crystal structure of enoyl-ACP reductase (FabK) from Streptococcus pneumoniae at 1.7 A resolution. There was one dimer per asymmetric unit. Each subunit formed a triose phosphate isomerase (TIM) barrel structure, and flavin mononucleotide (FMN) was bound as a cofactor in the active site. The overall structure was similar to the enoyl-ACP reductase (ER) of fungal fatty acid synthase and to 2-nitropropane dioxygenase (2-ND) from Pseudomonas aeruginosa, although there were some differences among these structures. We determined the crystal structure of FabK in complex with a phenylimidazole derivative inhibitor to envision the binding site interactions. The crystal structure reveals that the inhibitor binds to a hydrophobic pocket in the active site of FabK, and this is accompanied by induced-fit movements of two loop regions. The thiazole ring and part of the ureido moiety of the inhibitor are involved in a face-to-face pi-pi stacking interaction with the isoalloxazine ring of FMN. The side-chain conformation of the proposed catalytic residue, His144, changes upon complex formation. Lineweaver-Burk plots indicate that the inhibitor binds competitively with respect to NADH, and uncompetitively with respect to crotonoyl coenzyme A. We propose that the primary basis of the inhibitory activity is competition with NADH for binding to FabK, which is the first step of the two-step ping-pong catalytic mechanism.
Organizational Affiliation:
Pharmaceutical Research Center, Meiji Seika Kaisha, Ltd., Kohoku-ku, Yokohama 222-8567, Japan. [email protected]