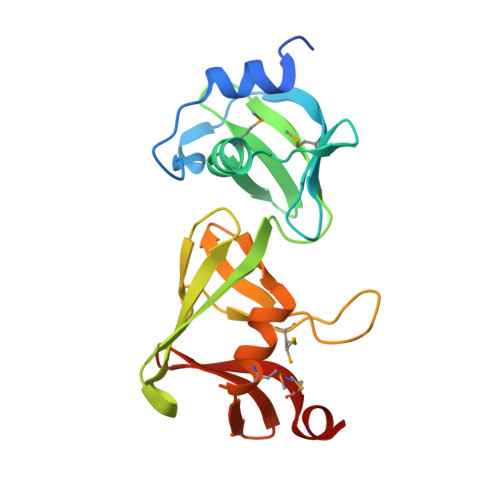
Structures of the first representatives of Pfam family PF06938 (DUF1285) reveal a new fold with repeated structural motifs and possible involvement in signal transduction.
Han, G.W., Bakolitsa, C., Miller, M.D., Kumar, A., Carlton, D., Najmanovich, R.J., Abdubek, P., Astakhova, T., Axelrod, H.L., Chen, C., Chiu, H.J., Clayton, T., Das, D., Deller, M.C., Duan, L., Ernst, D., Feuerhelm, J., Grant, J.C., Grzechnik, A., Jaroszewski, L., Jin, K.K., Johnson, H.A., Klock, H.E., Knuth, M.W., Kozbial, P., Krishna, S.S., Marciano, D., McMullan, D., Morse, A.T., Nigoghossian, E., Okach, L., Reyes, R., Rife, C.L., Sefcovic, N., Tien, H.J., Trame, C.B., van den Bedem, H., Weekes, D., Xu, Q., Hodgson, K.O., Wooley, J., Elsliger, M.A., Deacon, A.M., Godzik, A., Lesley, S.A., Wilson, I.A.(2010) Acta Crystallogr Sect F Struct Biol Cryst Commun 66: 1218-1225
- PubMed: 20944214
- DOI: https://doi.org/10.1107/S1744309109050416
- Primary Citation of Related Structures:
2RA9, 2RE3 - PubMed Abstract:
The crystal structures of SPO0140 and Sbal_2486 were determined using the semiautomated high-throughput pipeline of the Joint Center for Structural Genomics (JCSG) as part of the NIGMS Protein Structure Initiative (PSI). The structures revealed a conserved core with domain duplication and a superficial similarity of the C-terminal domain to pleckstrin homology-like folds. The conservation of the domain interface indicates a potential binding site that is likely to involve a nucleotide-based ligand, with genome-context and gene-fusion analyses additionally supporting a role for this family in signal transduction, possibly during oxidative stress.
Organizational Affiliation:
Stanford Synchrotron Radiation ightsource, SLAC National Accelerator Laboratory, Menlo Park, CA, USA.