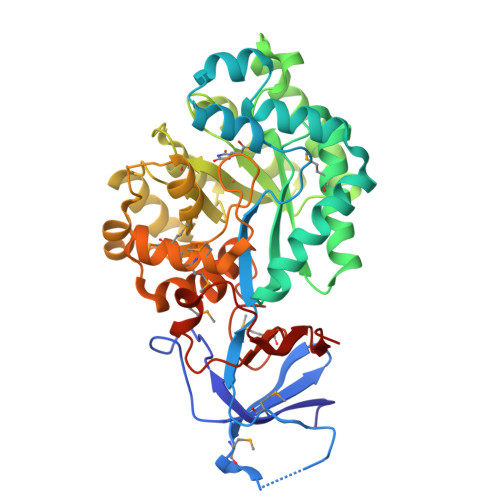
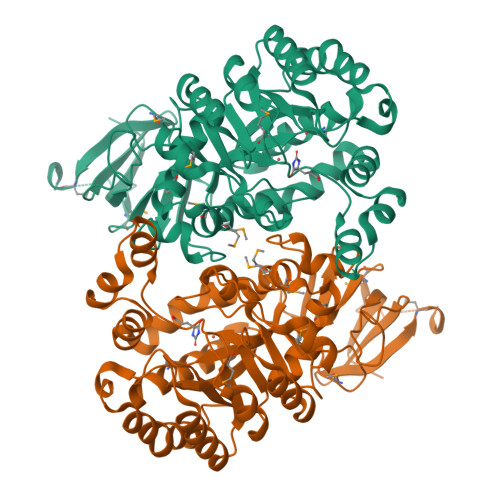
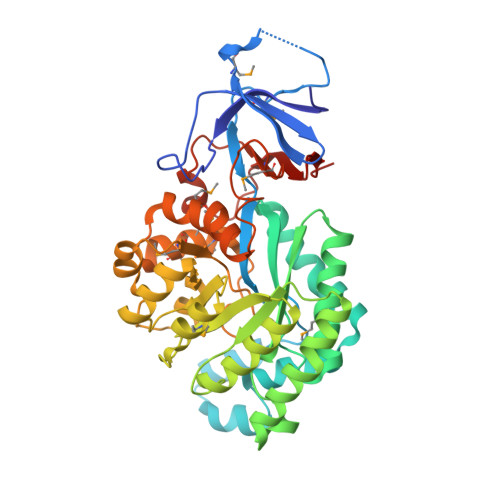
A common catalytic mechanism for proteins of the HutI family.
Tyagi, R., Eswaramoorthy, S., Burley, S.K., Raushel, F.M., Swaminathan, S.(2008) Biochemistry 47: 5608-5615
- PubMed: 18442260
- DOI: https://doi.org/10.1021/bi800180g
- Primary Citation of Related Structures:
2Q09 - PubMed Abstract:
Imidazolonepropionase (HutI) (imidazolone-5-propanote hydrolase, EC 3.5.2.7) is a member of the amidohydrolase superfamily and catalyzes the conversion of imidazolone-5-propanoate to N-formimino-L-glutamate in the histidine degradation pathway. We have determined the three-dimensional crystal structures of HutI from Agrobacterium tumefaciens (At-HutI) and an environmental sample from the Sargasso Sea Ocean Going Survey (Es-HutI) bound to the product [ N-formimino-L-glutamate (NIG)] and an inhibitor [3-(2,5-dioxoimidazolidin-4-yl)propionic acid (DIP)], respectively. In both structures, the active site is contained within each monomer, and its organization displays the landmark feature of the amidohydrolase superfamily, showing a metal ligand (iron), four histidines, and one aspartic acid. A catalytic mechanism involving His265 is proposed on the basis of the inhibitor-bound structure. This mechanism is applicable to all HutI forms.
Organizational Affiliation:
Biology Department, Brookhaven National Laboratory, Upton, New York 11973, USA.