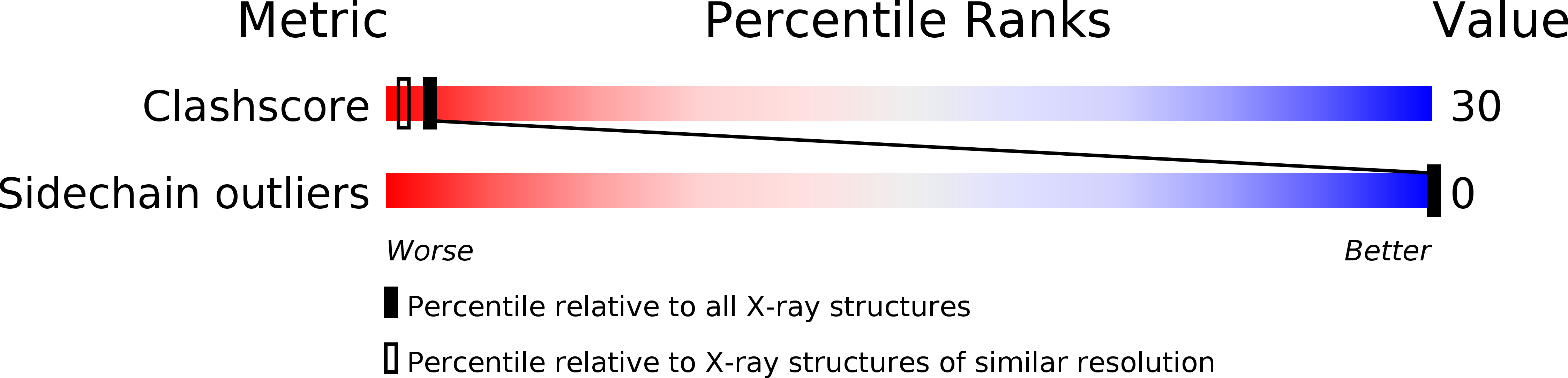An unusual buried polar cluster in a family of fungal lipases.
Derewenda, U., Swenson, L., Green, R., Wei, Y., Dodson, G.G., Yamaguchi, S., Haas, M.J., Derewenda, Z.S.(1994) Nat Struct Biol 1: 36-47
- PubMed: 7656005
- DOI: https://doi.org/10.1038/nsb0194-36
- Primary Citation of Related Structures:
1TIA - PubMed Abstract:
The stability of globular proteins arises largely from the burial of non-polar amino acids in their interior. These residues are efficiently packed to eliminate energetically unfavorable cavities. Contrary to these observations, high resolution X-ray crystallographic analyses of four homologous lipases from filamentous fungi reveal an alpha/beta fold which contains a buried conserved constellation of charged and polar side chains with associated cavities containing ordered water molecules. It is possible that this structural arrangement plays an important role in interfacial catalysis.
Organizational Affiliation:
Department of Biochemistry, University of Alberta, Edmonton, Canada.