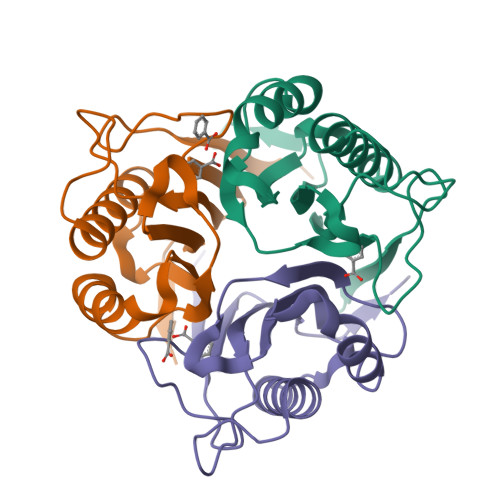
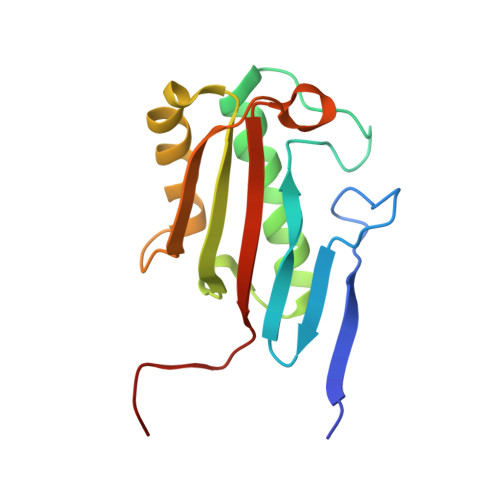
Crystal structure of Homo sapiens protein hp14.5.
Manjasetty, B.A., Delbruck, H., Pham, D.T., Mueller, U., Fieber-Erdmann, M., Scheich, C., Sievert, V., Bussow, K., Niesen, F.H., Weihofen, W., Loll, B., Saenger, W., Heinemann, U., Neisen, F.H.(2004) Proteins 54: 797-800
- PubMed: 14997576
- DOI: https://doi.org/10.1002/prot.10619
- Primary Citation of Related Structures:
1ONI
Organizational Affiliation:
Protein Structure Factory, c/o BESSY GmbH, Berlin, Germany.