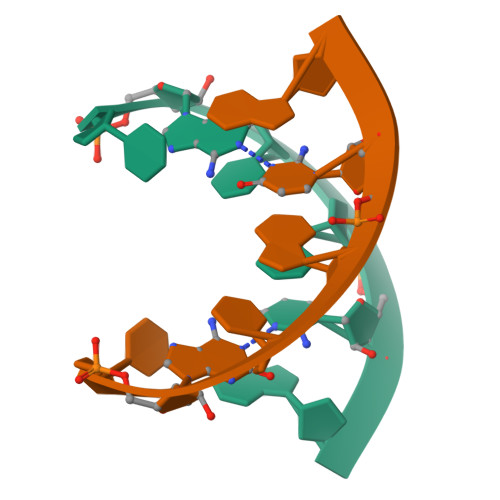
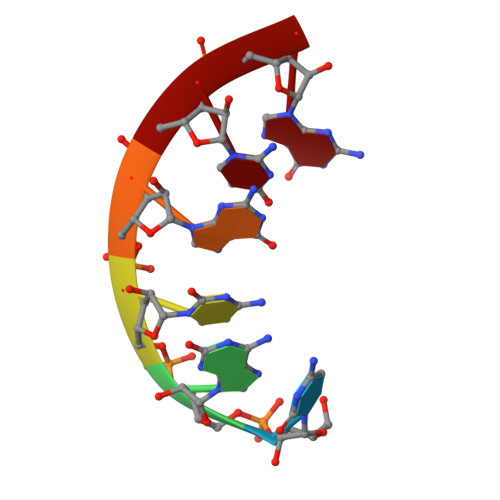
Stability and structure of RNA duplexes containing isoguanosine and isocytidine.
Chen, X., Kierzek, R., Turner, D.H.(2001) J Am Chem Soc 123: 1267-1274
- PubMed: 11456697
- DOI: https://doi.org/10.1021/ja002623i
- Primary Citation of Related Structures:
1G3A - PubMed Abstract:
Isoguanosine (iG) and isocytidine (iC) differ from guanosine (G) and cytidine (C), respectively, in that the amino and carbonyl groups are transposed. The thermodynamic properties of a set of iG, iC containing RNA duplexes have been measured by UV optical melting. It is found that iG-iC replacements usually stabilize duplexes, and the stabilization per iG-iC pair is sequence-dependent. The sequence dependence can be fit to a nearest-neighbor model in which the stabilities of iG--iC pairs depend on the adjacent iG--iC or G--C pairs. For 5'-CG-3'/3'-GC-5' and 5'-GG-3'/3'-CC-5' nearest neighbors, the free energy differences upon iG-iC replacement are smaller than 0.2 kcal/mol at 37 degrees C, regardless of the number of replacements. For 5'-GC-3'/3'-CG-5', however, each iG--iC replacement adds 0.6 kcal/mol stabilizing free energy at 37 degrees C. Stacking propensities of iG and iC as unpaired nucleotides at the end of a duplex are similar to those of G and C. An NMR structure is reported for r(CiGCGiCG)(2) and found to belong to the A-form family. The structure has substantial deviations from standard A-form but is similar to published NMR and/or crystal structures for r(CGCGCG)(2) and 2'-O-methyl (CGCGCG)(2). These results provide benchmarks for theoretical calculations aimed at understanding the fundamental physical basis for the thermodynamic stabilities of nucleic acid duplexes.
Organizational Affiliation:
Department of Chemistry, University of Rochester, Rochester, New York 14627-0216, USA.