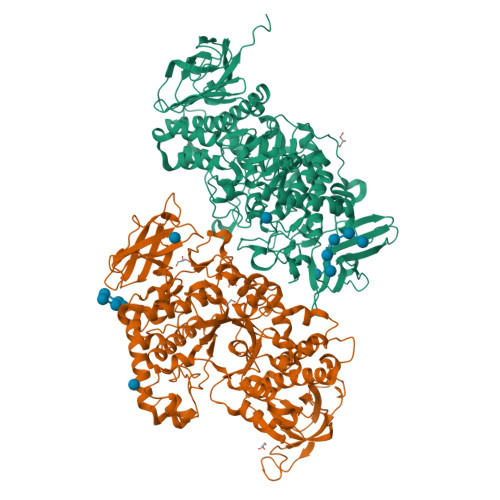
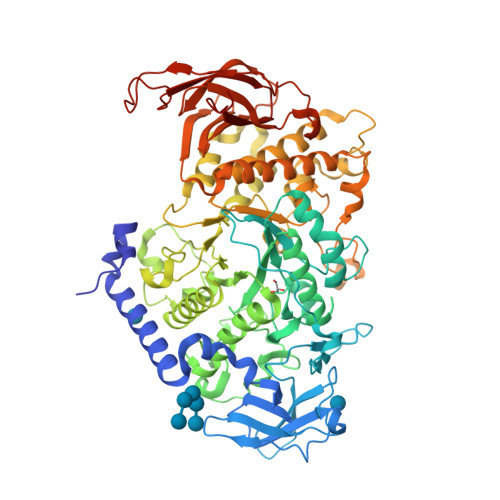
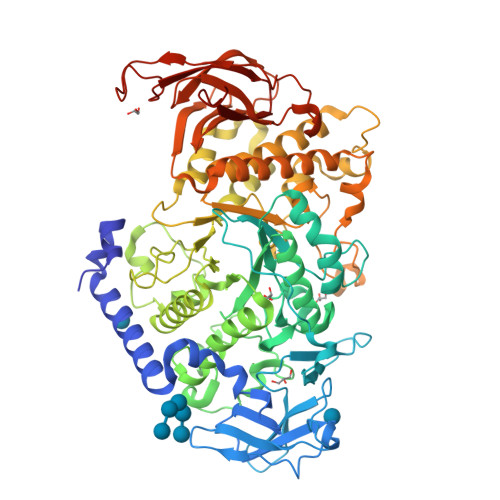
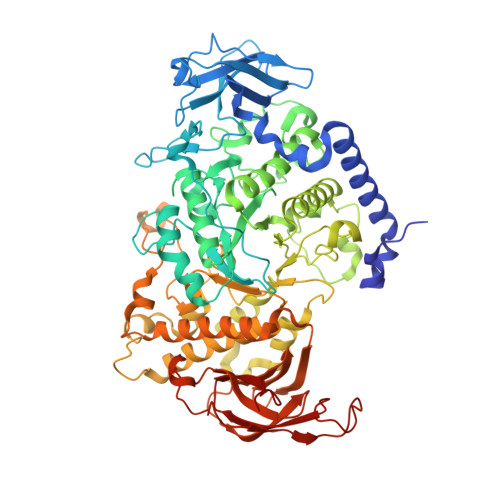
Crystal structure of the rice branching enzyme I (BEI) in complex with maltopentaose.
Chaen, K., Noguchi, J., Omori, T., Kakuta, Y., Kimura, M.(2012) Biochem Biophys Res Commun 424: 508-511
- PubMed: 22771800
- DOI: https://doi.org/10.1016/j.bbrc.2012.06.145
- Primary Citation of Related Structures:
3VU2 - PubMed Abstract:
Starch branching enzyme (SBE) catalyzes the cleavage of α-1,4-linkages and the subsequent transfer of α-1,4 glucan to form an α-1,6 branch point in amylopectin. We determined the crystal structure of the rice branching enzyme I (BEI) in complex with maltopentaose at a resolution of 2.2Å. Maltopentaose bound to a hydrophobic pocket formed by the N-terminal helix, carbohydrate-binding module 48 (CBM48), and α-amylase domain. In addition, glucose moieties could be observed at molecular surfaces on the N-terminal helix (α2) and CBM48. Amino acid residues involved in the carbohydrate bindings are highly conserved in other SBEs, suggesting their generally conserved role in substrate binding for SBEs.
Organizational Affiliation:
Laboratory of Structural Biology, Graduate School of Systems Life Sciences, Kyushu University, Hakozaki 6-10-1, Fukuoka 812-8581, Japan.