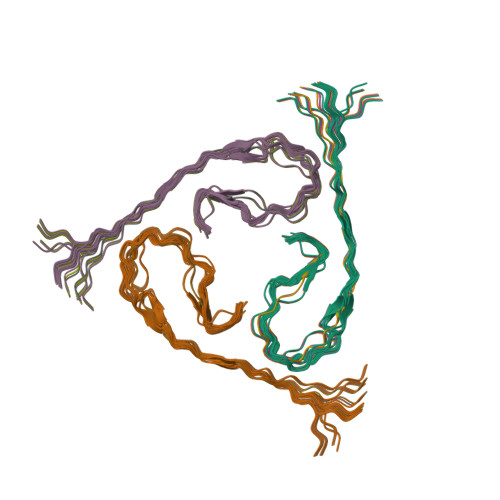
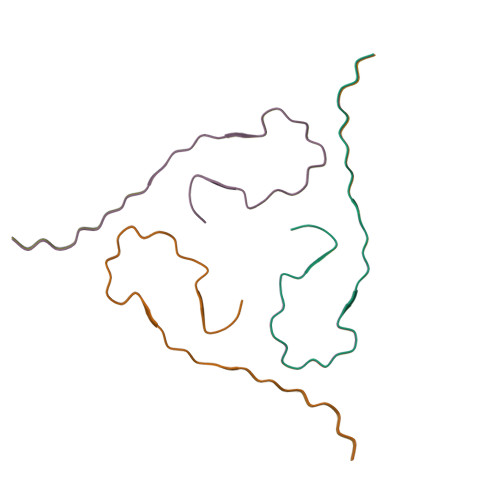
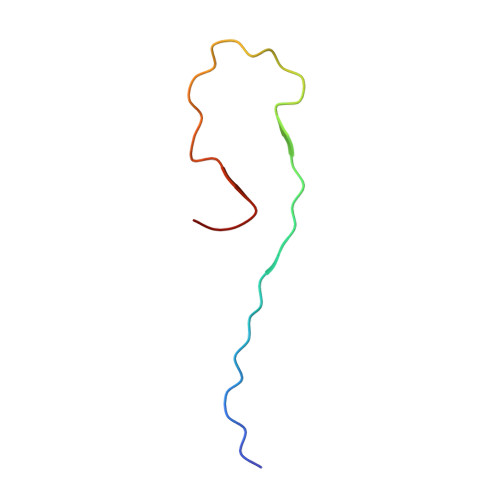
Molecular Structure of beta-Amyloid Fibrils in Alzheimer's Disease Brain Tissue.
Lu, J.X., Qiang, W., Yau, W.M., Schwieters, C.D., Meredith, S.C., Tycko, R.(2013) Cell 154: 1257-1268
- PubMed: 24034249
- DOI: https://doi.org/10.1016/j.cell.2013.08.035
- Primary Citation of Related Structures:
2M4J - PubMed Abstract:
In vitro, β-amyloid (Aβ) peptides form polymorphic fibrils, with molecular structures that depend on growth conditions, plus various oligomeric and protofibrillar aggregates. Here, we investigate structures of human brain-derived Aβ fibrils, using seeded fibril growth from brain extract and data from solid-state nuclear magnetic resonance and electron microscopy. Experiments on tissue from two Alzheimer's disease (AD) patients with distinct clinical histories showed a single predominant 40 residue Aβ (Aβ40) fibril structure in each patient; however, the structures were different from one another. A molecular structural model developed for Aβ40 fibrils from one patient reveals features that distinguish in-vivo- from in-vitro-produced fibrils. The data suggest that fibrils in the brain may spread from a single nucleation site, that structural variations may correlate with variations in AD, and that structure-specific amyloid imaging agents may be an important future goal.
Organizational Affiliation:
Laboratory of Chemical Physics, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD 20892-0520, USA.