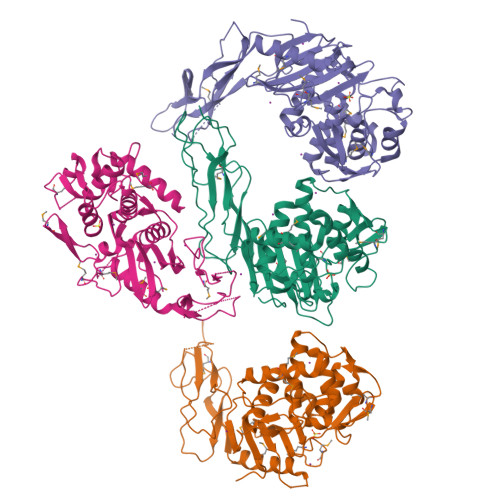
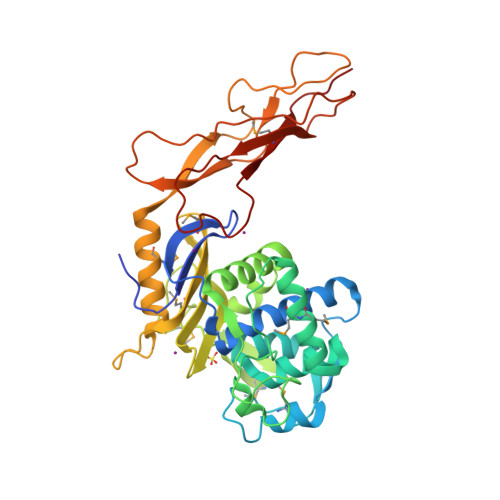
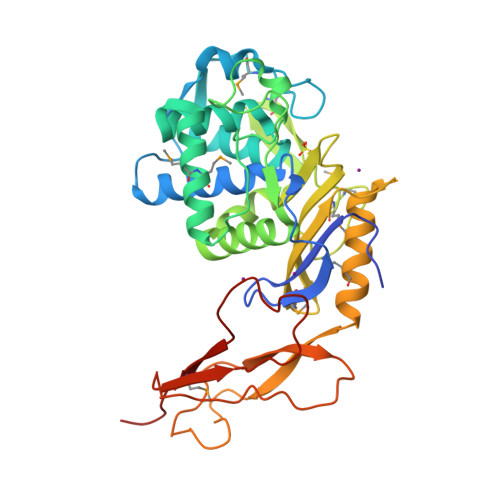
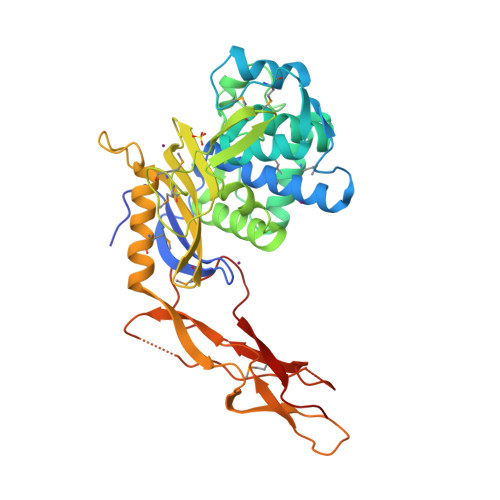
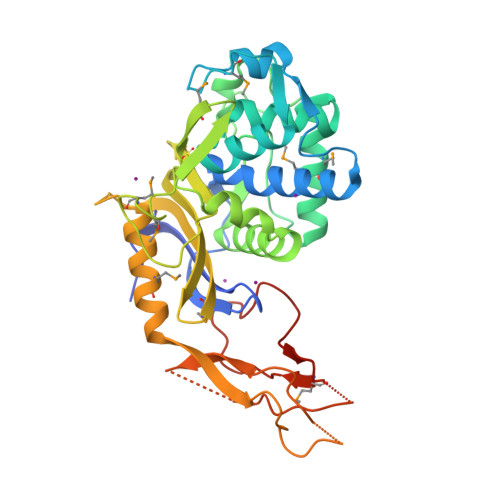
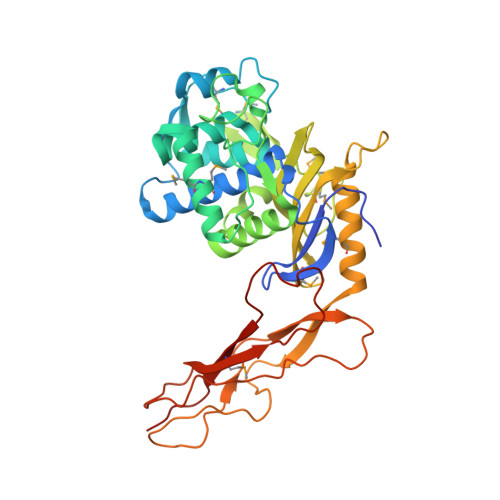
Crystal structure of a peptidoglycan synthesis regulatory factor (PBP3) from Streptococcus pneumoniae
Morlot, C., Pernot, L., Le Gouellec, A., Di Guilmi, A.M., Vernet, T., Dideberg, O., Dessen, A.(2005) J Biological Chem 280: 15984-15991
- PubMed: 15596446
- DOI: https://doi.org/10.1074/jbc.M408446200
- Primary Citation of Related Structures:
1XP4 - PubMed Abstract:
Penicillin-binding proteins (PBPs) are membrane-associated enzymes which perform critical functions in the bacterial cell division process. The single d-Ala,d-Ala (d,d)-carboxypeptidase in Streptococcus pneumoniae, PBP3, has been shown to play a key role in control of availability of the peptidoglycal substrate during cell growth. Here, we have biochemically characterized and solved the crystal structure of a soluble form of PBP3 to 2.8 A resolution. PBP3 folds into an NH(2)-terminal, d,d-carboxypeptidase-like domain, and a COOH-terminal, elongated beta-rich region. The carboxypeptidase domain harbors the classic signature of the penicilloyl serine transferase superfamily, in that it contains a central, five-stranded antiparallel beta-sheet surrounded by alpha-helices. As in other carboxypeptidases, which are present in species whose peptidoglycan stem peptide has a lysine residue at the third position, PBP3 has a 14-residue insertion at the level of its omega loop, a feature that distinguishes it from carboxypeptidases from bacteria whose peptidoglycan harbors a diaminopimelate moiety at this position. PBP3 performs substrate acylation in a highly efficient manner (k(cat)/K(m) = 50,500 M(-1) x s(-1)), an event that may be linked to role in control of pneumococcal peptidoglycan reticulation. A model that places PBP3 poised vertically on the bacterial membrane suggests that its COOH-terminal region could act as a pedestal, placing the active site in proximity to the peptidoglycan and allowing the protein to "skid" on the surface of the membrane, trimming pentapeptides during the cell growth and division processes.
Organizational Affiliation:
Laboratoire de Cristallographie Macromoléculaire and Laboratoire d'Ingénierie des Macromolécules, Institut de Biologie Structurale Jean-Pierre Ebel (CNRS/CEA/UJF), 41 rue Jules Horowitz, Grenoble 38027, France.