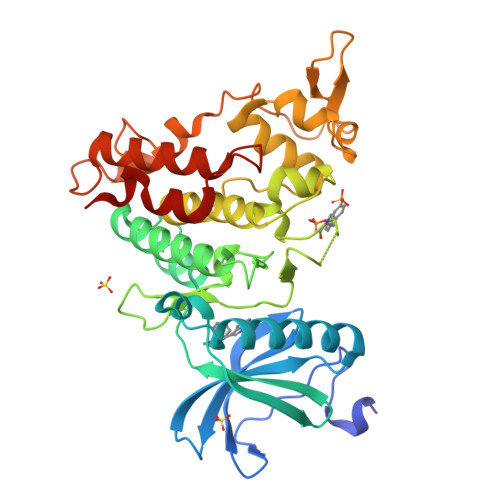
Mining Public Domain Data to Develop Selective DYRK1A Inhibitors.
Henderson, S.H., Sorrell, F., Bennett, J., Hanley, M.T., Robinson, S., Hopkins Navratilova, I., Elkins, J.M., Ward, S.E.(2020) ACS Med Chem Lett 11: 1620-1626
- PubMed: 32832032
- DOI: https://doi.org/10.1021/acsmedchemlett.0c00279
- Primary Citation of Related Structures:
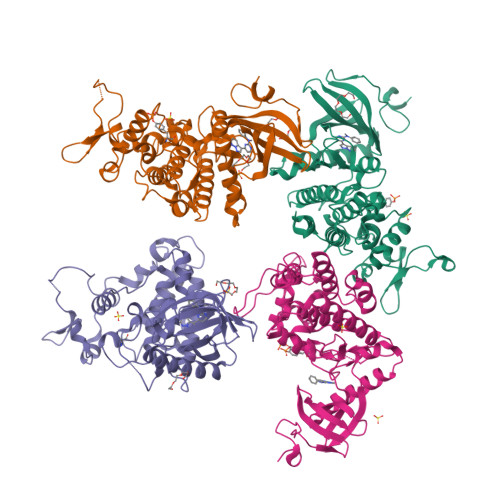
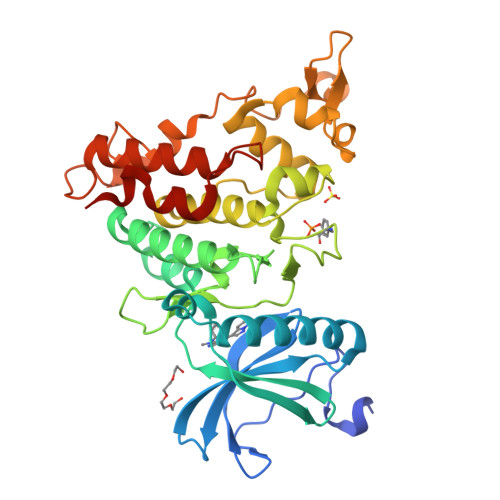
6S1I - PubMed Abstract:
Kinases represent one of the most intensively pursued groups of targets in modern-day drug discovery. Often it is desirable to achieve selective inhibition of the kinase of interest over the remaining ∼500 kinases in the human kinome. This is especially true when inhibitors are intended to be used to study the biology of the target of interest. We present a pipeline of open-source software that analyzes public domain data to repurpose compounds that have been used in previous kinase inhibitor development projects. We define the dual-specificity tyrosine-regulated kinase 1A (DYRK1A) as the kinase of interest, and by addition of a single methyl group to the chosen starting point we remove glycogen synthase kinase β (GSK3β) and cyclin-dependent kinase (CDK) inhibition. Thus, in an efficient manner we repurpose a GSK3β/CDK chemotype to deliver 8b , a highly selective DYRK1A inhibitor.
Organizational Affiliation:
Sussex Drug Discovery Centre, University of Sussex, Brighton BN1 9RH, U.K.