Discovery of a Kelch-like ECH-associated protein 1-inhibitory tetrapeptide and its structural characterization
Sogabe, S., Sakamoto, K., Kamada, Y., Kadotani, A., Fukuda, Y., Sakamoto, J.(2017) Biochem Biophys Res Commun 486: 620-625
- PubMed: 28315327
- DOI: https://doi.org/10.1016/j.bbrc.2017.03.038
- Primary Citation of Related Structures:
5X54 - PubMed Abstract:
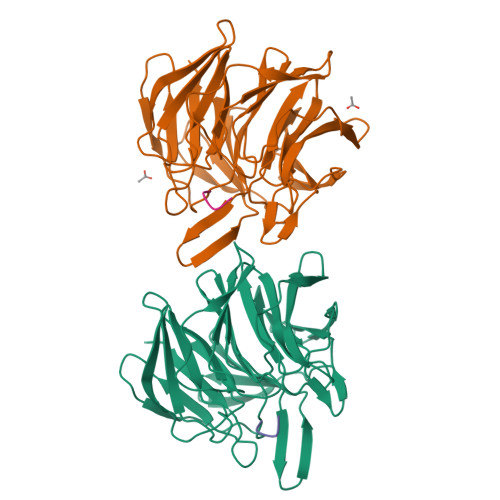
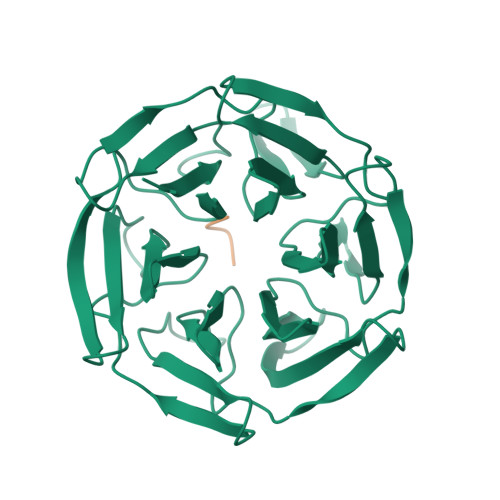
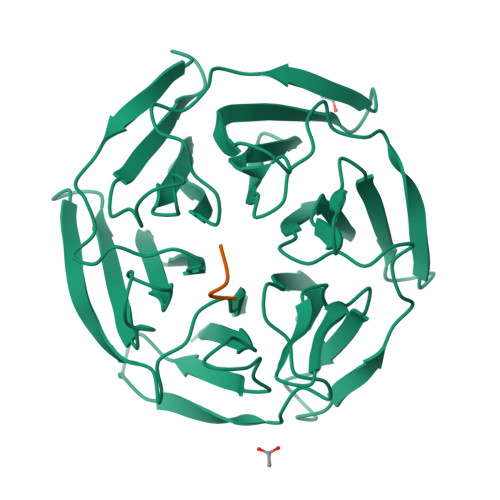
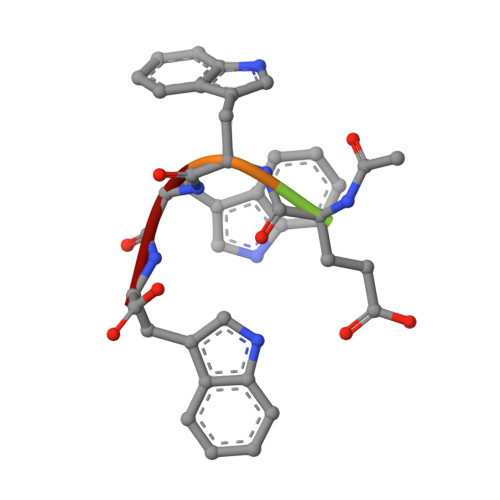
Keap1 constitutively binds to the transcription factor Nrf2 to promote its degradation, resulting in negative modulation of genes involved in cellular protection against oxidative stress. Keap1 is increasingly recognized as an attractive target for treating diseases involving oxidative stress, including cancer, atherosclerosis, diabetes, arthritis, and neurodegeneration. We used phage-display peptide screening to identify a tetrapeptide showing moderate binding affinity, which inhibits the interaction between Nrf2 and Keap1. The tetrapeptide does not include an ETGE motif, which is a commonly found consensus sequence in known peptidic inhibitors. In addition to affinity parameters, IC 50 , K D , and thermodynamic parameters, the crystal structure of the complex was determined to elucidate the binding conformation. The binding interactions resemble those of known small-molecule inhibitors as opposed to those of substrates and peptidic inhibitors. Although the tetrapeptide's affinity is not very high, our results may help facilitate the designing of small-molecule inhibitors during lead generation in drug discovery.
Organizational Affiliation:
Pharmaceutical Research Division, Takeda Pharmaceutical Company Limited, 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Electronic address: satoshi.sogabe@takeda.com.