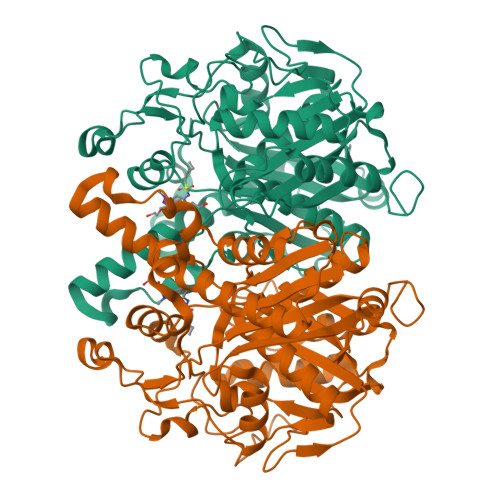
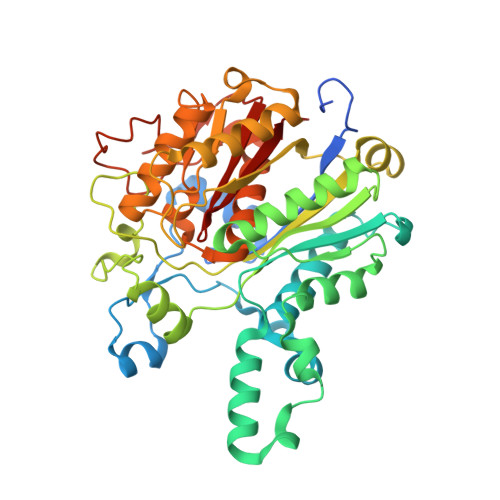
Identification of KasA as the cellular target of an anti-tubercular scaffold.
Abrahams, K.A., Chung, C.W., Ghidelli-Disse, S., Rullas, J., Rebollo-Lopez, M.J., Gurcha, S.S., Cox, J.A., Mendoza, A., Jimenez-Navarro, E., Martinez-Martinez, M.S., Neu, M., Shillings, A., Homes, P., Argyrou, A., Casanueva, R., Loman, N.J., Moynihan, P.J., Lelievre, J., Selenski, C., Axtman, M., Kremer, L., Bantscheff, M., Angulo-Barturen, I., Izquierdo, M.C., Cammack, N.C., Drewes, G., Ballell, L., Barros, D., Besra, G.S., Bates, R.H.(2016) Nat Commun 7: 12581-12581
- PubMed: 27581223
- DOI: https://doi.org/10.1038/ncomms12581
- Primary Citation of Related Structures:
5LD8 - PubMed Abstract:
Phenotypic screens for bactericidal compounds are starting to yield promising hits against tuberculosis. In this regard, whole-genome sequencing of spontaneous resistant mutants generated against an indazole sulfonamide (GSK3011724A) identifies several specific single-nucleotide polymorphisms in the essential Mycobacterium tuberculosis β-ketoacyl synthase (kas) A gene. Here, this genomic-based target assignment is confirmed by biochemical assays, chemical proteomics and structural resolution of a KasA-GSK3011724A complex by X-ray crystallography. Finally, M. tuberculosis GSK3011724A-resistant mutants increase the in vitro minimum inhibitory concentration and the in vivo 99% effective dose in mice, establishing in vitro and in vivo target engagement. Surprisingly, the lack of target engagement of the related β-ketoacyl synthases (FabH and KasB) suggests a different mode of inhibition when compared with other Kas inhibitors of fatty acid biosynthesis in bacteria. These results clearly identify KasA as the biological target of GSK3011724A and validate this enzyme for further drug discovery efforts against tuberculosis.
Organizational Affiliation:
Institute of Microbiology and Infection, School of Biosciences, University of Birmingham, Edgbaston, Birmingham B15 2TT, UK.