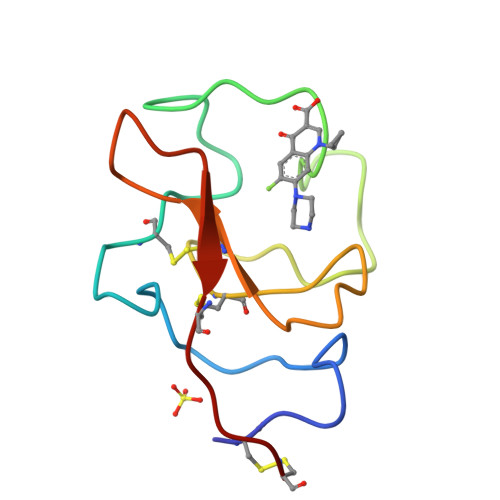
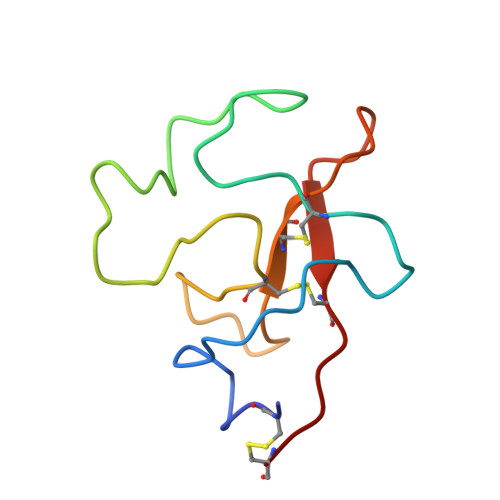
Small Molecules Used to Decipher the Pathophysiological Roles of the Kringle Domains Kiv-7, - 10 and Kv of Apolipoprotein(A)
Sandmark, J., Althage, M., Andersson, G.M.K., Antonsson, T., Blaho, S., Bodin, C., Bostrom, J., Chen, Y., Dahlen, A., Eriksson, P.O., Evertsson, E., Fex, T., Fjellstrom, O., Gustafsson, D., Hallberg, C., Hicks, R., Jarkvist, E., Johansson, C., Kalies, I., Kang, D., Svalstedt Karlsson, B., Kartberg, F., Legnehed, A., Lindqvist, A.M., Martinsson, S.A., Moberg, A., Petersson, A.U., Ridderstrom, M., Thelin, A., Tigerstrom, A., Vinblad, J., Xu, B., Knecht, W.To be published.