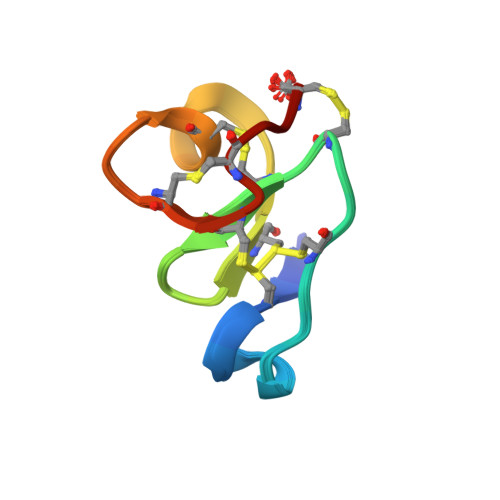
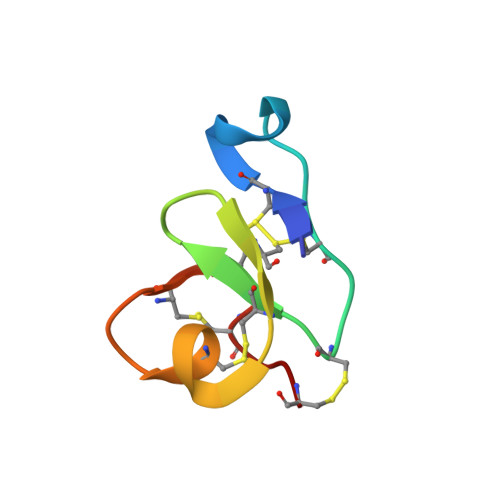
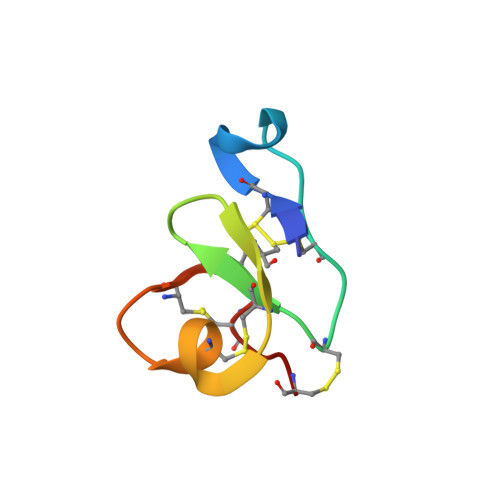
Solution structure of a defense peptide from wheat with a 10-cysteine motif.
Dubovskii, P.V., Vassilevski, A.A., Slavokhotova, A.A., Odintsova, T.I., Grishin, E.V., Egorov, T.A., Arseniev, A.S.(2011) Biochem Biophys Res Commun 411: 14-18
- PubMed: 21704019
- DOI: https://doi.org/10.1016/j.bbrc.2011.06.058
- Primary Citation of Related Structures:
2LB7 - PubMed Abstract:
Hevein, a well-studied lectin from the rubber tree Hevea brasiliensis, is the title representative of a broad family of chitin-binding polypeptides. WAMP-1a, a peptide isolated from the wheat Triticum kiharae, shares considerable similarity with hevein. The peptide possesses antifungal, antibacterial activity and is thought to play an important role in the defense system of wheat. Importantly, it features a substitution of the conserved serine residue to glycine reducing its carbohydrate-binding capacity. We used NMR spectroscopy to derive the spatial structure of WAMP-1a in aqueous solution. Notably, the mutation was found to strengthen amphiphilicity of the molecule, associated with its mode of action, an indication of the hevein domain multi-functionality. Both primary and tertiary structure of WAMP-1a suggest its evolutionary origin from the hevein domain of plant chitinases.
Organizational Affiliation:
Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, 16/10 Miklukho-Maklaya Str., 117997 Moscow, Russian Federation. peter@nmr.ru