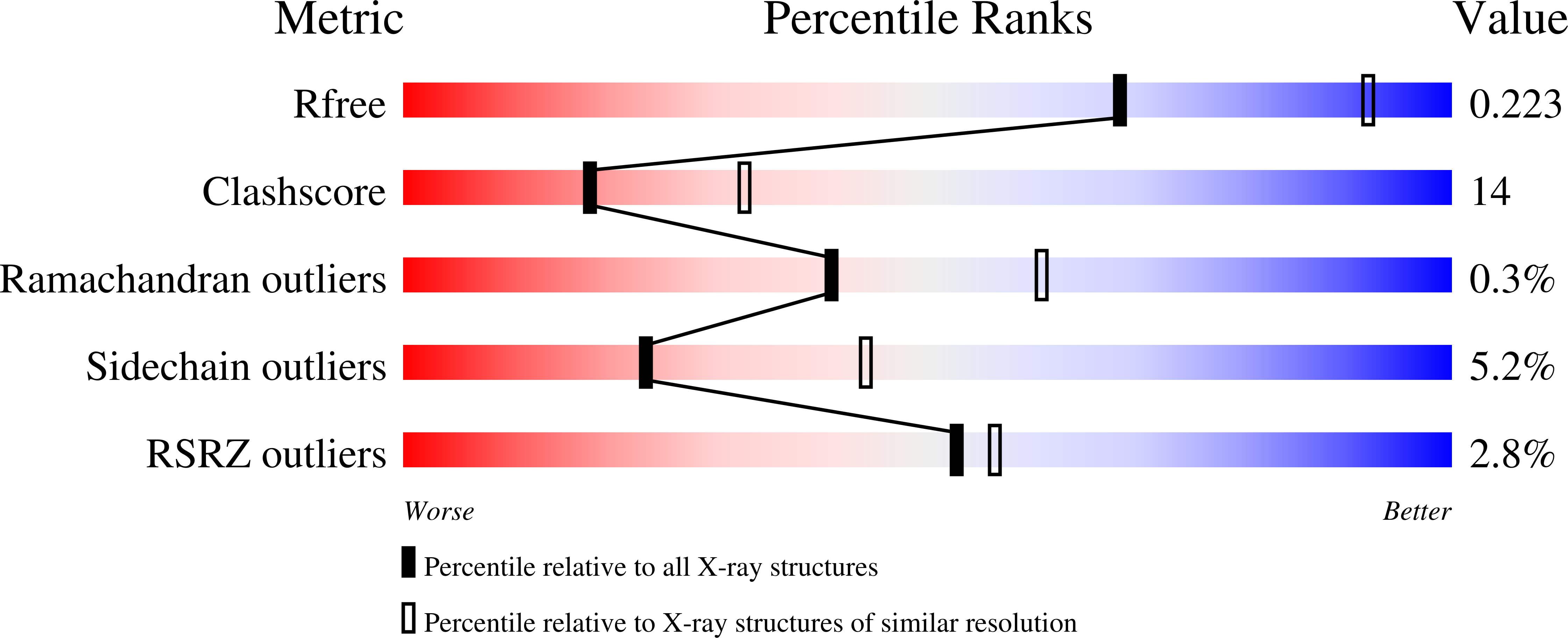
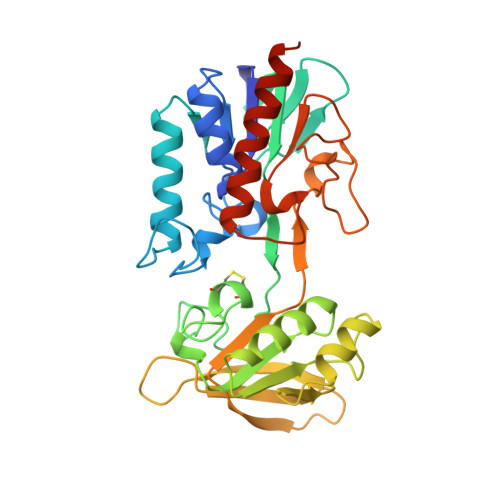
Crystal structure of Arabidopsis thaliana NADPH dependent thioredoxin reductase at 2.5 A resolution.
Dai, S., Saarinen, M., Ramaswamy, S., Meyer, Y., Jacquot, J.P., Eklund, H.(1996) J Mol Biol 264: 1044-1057
- PubMed: 9000629
- DOI: https://doi.org/10.1006/jmbi.1996.0695
- Primary Citation of Related Structures:
1VDC - PubMed Abstract:
Thioredoxin exists in all organisms and is responsible for the hydrogen transfer to important enzymes for ribonucleotide reduction and the reduction of methionine sulphoxide and sulphate. Thioredoxins have also been shown to regulate enzyme activity in plants and are also involved in the regulation of transcription factors and several other regulatory activities. Thioredoxin is reduced by the flavoenzyme thioredoxin reductase using NADPH. We have now determined the first structure of a eukaryotic thioredoxin reductase, from the plant Arabidopsis thaliana, at 2.5 A resolution. The dimeric A. thaliana thioredoxin reductase is structurally similar to that of the Escherichia coli enzyme, and most differences occur in the loops. Because the plant and E. coli enzymes have the same architecture, with the same dimeric structure and the same position of the redox active disulphide bond, a similar mechanism that involves very large domain rotations is likely for the two enzymes. The subunit is divided into two domains, one that binds FAD and one that binds NADPH. The relative positions of the domains in A. thaliana thioredoxin reductase differ from those of the E. coli reductase. When the FAD domains are superimposed, the NADPH domain of A. thaliana thioredoxin reductase must be rotated by 8 degrees to superimpose on the corresponding domain of the E. coli enzyme. The domain rotation we now observe is much smaller than necessary for the thioredoxin reduction cycle.
Organizational Affiliation:
Department of Molecular Biology, Swedish University of Agricultural Sciences, Uppsala, Sweden.